Int J Drug Res Clin. 2:e5.
doi: 10.34172/ijdrc.2024.e5
Original Article
Spleen and Pancreatic Tissue Change after Genetically Modified Soybean Oil Consumption Among Male Wistar Rats
Horiyie Taheri 1  , Mehran Mesgari-Abbasi 2, Mahdieh Abbasalizad-Farhangi 1, *
, Mehran Mesgari-Abbasi 2, Mahdieh Abbasalizad-Farhangi 1, * 
Author information:
1Department of Community Nutrition, Faculty of Nutrition, Tabriz University of Medical Sciences, Tabriz, Iran
2Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Background:
Over the past 20 years, the increased consumption of transgenic products for humans and animals led to the conduction of nutritional studies in this regard. However, these studies were limited, and they did not find a definitive answer to the possible health hazards of transgenic products. Therefore, this study was designed to evaluate the effects of a diet containing transgenic soybean oil on rats.
Methods:
Accordingly, male Wistar rats (N=6/group) were given a nutritionally moderate purified diet with 10% genetically modified soybean oil for 90 days. Two control groups receiving non-genetically modified soybean oil and a standardized diet were also enrolled. One-way analysis of variance (ANOVA) followed by Tuky post hoc analysis was used to compare the values between groups and to detect the effects of transgenic soybean oil.
Results:
Rats fed on transgenic soybean oil demonstrated several histologic changes in pancreas tissues, including changes in severe congestion, the presence of inflammatory cells, and changes in the Langerhans islands. However, no changes were observed in the spleen, except for negligible congestion in all treatment groups. Regarding blood indicators, hemoglobin levels in the transgenic soybean oil group decreased compared to the other two groups (P<0.05).
Conclusion:
According to our results, a 90-day treatment with transgenic soy-based oil caused significant organ changes in the pancreas tissue of rats. Further studies evaluating the long-term effects are also needed to better elucidate these effects.
Keywords: Food, Genetically modified, Soybean oil, Rats, Spleen, Pancreas
Copyright and License Information
© 2024 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Taheri H, Mesgari-Abbasi M, Abbasalizad-Farhangi M. Spleen and pancreatic tissue change after genetically modified soybean oil consumption among male wistar rats. Int J Drug Res Clin. 2024; 2: e5. doi: 10.34172/ijdrc.2024.e5
Introduction
In the last two decades, genetic engineering using modern biotechnology has led to the production of genetically modified products in the world. In this way, new genes are transmitted to the desired species using the recombinant DNA to produce a favorable feature, while its transmission is not possible by traditional methods.1 These new genes come from a bacterium called Bacillus thuringiensis (Bt). Naturally, this bacterium makes a protein that is noxious to pests and insects. The gene in the bacterium makes a protein called “Bt” gene2 with resistance to herbicides and pests as its most important property. The most popular transgenic food products are soybeans, corn, canola, and cotton with a special role in the food chain. Despite the wide acceptance of biotechnology at the farm level, its acceptance by the consumer is still uncertain.1,3,4
Animal feeding studies of transgenic products are useful for assessing their safety.5 Several experimental models displayed serious health hazards associated with genetically modified (GM) foods, including infertility, immune problems, accelerated aging, insulin regulation, and changes in vital organs and the gastrointestinal system.1,5-10 Several short or medium-term experimental studies exhibited the possible health hazards of GM foods.11,12 Health risks of GM foods arise from the inserted gene and their expressed protein as well as the possible disruption of natural genes in the manipulated organism.13 Toxic effects of commercialized GM soy and maize against vital organs,14-16 structural and molecular modifications in different organs and tissues of GM-fed animals,6,8 expanded spleen, possible spoiled spleen function, and hematological changes were reported in experimental models feeding GM foods.17 Ahrorovna reported morphological changes in the spleen of rats under the conditions of GM organism use which was characterized by an increase in size and weight and changes in the spleen structure.18 Moreover, negative effects of GM maize on liver and kidney function and tissue of rats were reported in Key et al and Malatesta and colleagues’ studies.19,20 In addition, it is believed that the consumption of these GM foods may lead to the development of diseases that are immune to antibiotics.21,22 The present study examined the effects of GM soybean oil on histological and biochemical features of the pancreas and spleen of 2-month-old male rats.
Materials and Methods
Diet Formulation
The composition of all diets is presented in Table 1. The GM soybean oil (GM oil) and non-GM soybean oil were prepared, and meals were provided by mixing 10% of both types of oils. The major nutritional contents of the laboratory diet were 22% protein, 3.48% fat, and 3.71% fiber.
Table 1.
Diet Formulation (%) for Treatment Groups
|
Ingredients
|
Standard
|
Control
|
GM
|
| Protein (g) |
22.5-23.5 |
22.5-23.5 |
22.5-23.5 |
| Fat (g) |
3.5 |
13.5 |
13.5 |
| Carbohydrate (g) |
52 |
52 |
52 |
| Fiber (g) |
4-5 |
4-5 |
4-5 |
| Ash (%) |
Maximum 10 |
Maximum 10 |
Maximum 10 |
| Calcium (g) |
0.95-1 |
0.95-1 |
0.95-1 |
| Phosphorus (g) |
0.65-7 |
0.65-7 |
0.65-7 |
| Salt (g) |
0.5-0.55 |
0.5-0.55 |
0.5-0.55 |
| Moisture (%) |
Maximum 10 |
Maximum 10 |
Maximum 10 |
| Lysine (g) |
1.15-1.2 |
1.15-1.2 |
1.15-1.2 |
| Methionine (g) |
0.33-0.37 |
0.33-0.37 |
0.33-0.37 |
| Methionine + (g) Cysteine |
0.63-0.65 |
0.63-0.65 |
0.63-0.65 |
| Threonine (g) |
0.73-0.75 |
0.73-0.75 |
0.73-0.75 |
| Tryptophan (g) |
0.25-0.32 |
0.25-0.32 |
0.25-0.32 |
| Energy (kcal ME/g) |
350 |
400 |
400 |
Note. GM: Genetically modified.
Animals and Housing
Eighteen male Wistar rats aged two months, with an average body weight of 195 ± 5 g were prepared from the animal house of Pasteur Research Institute, Tehran, Iran. The animals were adapted for one week on a normal diet. Then, the animals were subdivided and randomized into 3 groups (6 animals per group), feeding the 10% GM-oil diet group, the 10% non-GM-oil diet group, and the standard pellet diet group for 13 weeks. Three animal groups were kept in standard cages (6/cages) and under standard conditions. Temperature was retained at 22–25 °C, relative humidity was 55%–60% during the experience, and a 12-hour light/dark cycle was maintained. Diet and freshwater were prepared ad libitum. During the experimental period, general condition was checked daily, body weight was recorded weekly, and dietary consumptions were measured every 2 days. At the end of the study, all animals were anaesthetized by carbon dioxide inhalation and killed by exsanguinations for gross and histological examinations.
Hematologic Assay
At the end of the study, whole blood from the heart was collected with anticoagulant and analyzed for complete blood cell count (CBC) such as white blood cell count (WBC), red blood cell count (RBC), hematocrit (HCT), hemoglobin concentration (HC), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), hemoglobin distribution width (HDW), red cell distribution width (RDW), platelet distribution width (PDW), total platelet mass (PCT), blood platelet count (PLT), mean platelet volume (MPV), neutrophils, lymphocytes, eosinophils, basophils, and monocytes, and large unstained cells (LUC). These statistics were measured with a hematology analyzer set (H1Model, Auto-Tek-Kion Co., USA).
Histological Examination and Organ Weights
At the end of the experimental period, immune-related organs, liver, and kidney were weighed, and then a full set of tissues was collected. Tissues were put instantly into a 10% neutral buffered formalin for stabilization. The selected tissues were processed, placed in paraffin, sectioned (approximately 4 mm), and stained with hematoxylin and eosin using a typical and standard histological technique.23 Then, they were observed with a light microscope (Olympus, Japan).
Statistical Analysis
Statistical analyses were carried out by the SPSS software (Version 24, SPSS Inc., Chicago, IL, USA). Statistical comparisons of body weight, food consumption, clinical biochemistry, and organ weights between three group were performed by one-way analysis of variance (ANOVA) followed by Tukey post hoc analysis, and an independent samples t test was performed when appropriate. Differences were considered significant at P < 0.05, and data for each variable were expressed as mean ± standard error (SE).
Results
Changes in body weight and food intake are presented in Figures 1 and 2, respectively, with no significant changes in weight or food intake. The absolute mean organ weights are illustrated in Table 2. The results indicated no significant differences in organ weights of rats in all groups. However, the spleen weight was higher compared to other study groups. Moreover, the findings of the hematologic assessment of treatment groups are presented in Table 3, showing no statistically significant difference in hematologic parameters among treatment groups. Only, hemoglobin concentrations in the standard group were slightly higher than those in the other two groups (P < 0.001), while GM-fed rats tended to show lower levels of MCH, WBC, neutrophil, and lymphocyte compared to other treatment groups. In contrast, this group experienced a higher level of platelet compared to the other groups. Histologic derangements were observed in the pancreastissueof the GM soybean oil group. Various levels of destruction were also observed in this group, including severe congestion in the exocrine and endocrine section, the presence of inflammatory cells in the focal region of the exocrine (pancreatitis), vascular congestion, and a reduced number of Langerhans islands (Figure 3). In terms of changes in spleen tissue, no significant difference was observed between treatment groups except for slight congestion (Figure 4).
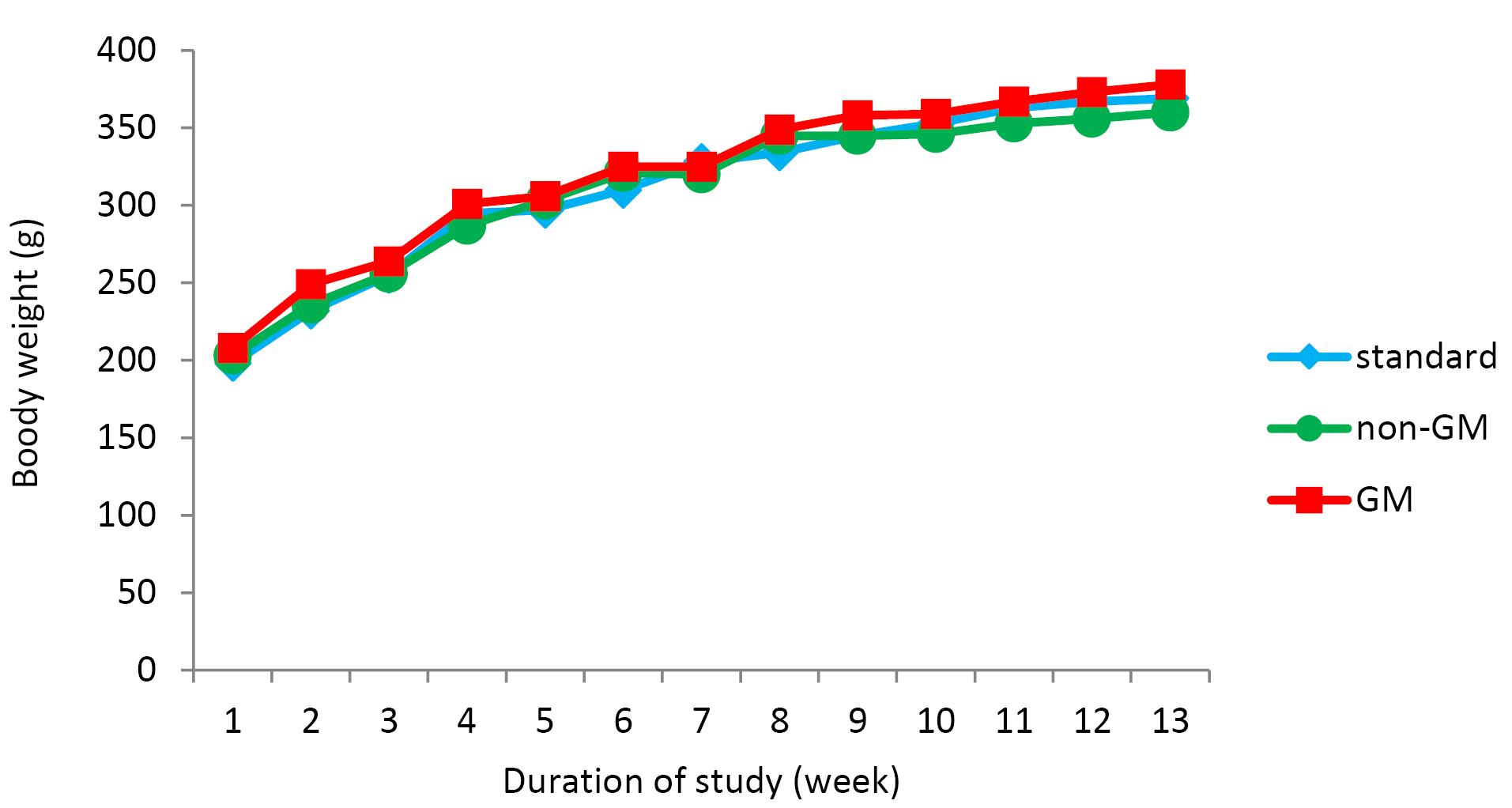
Figure 1.
Growth Curves Based on Weekly Measurements of Body Weight During the Study. Note. GM: Genetically modified. The curves show group means based on 6 rats /group
.
Growth Curves Based on Weekly Measurements of Body Weight During the Study. Note. GM: Genetically modified. The curves show group means based on 6 rats /group
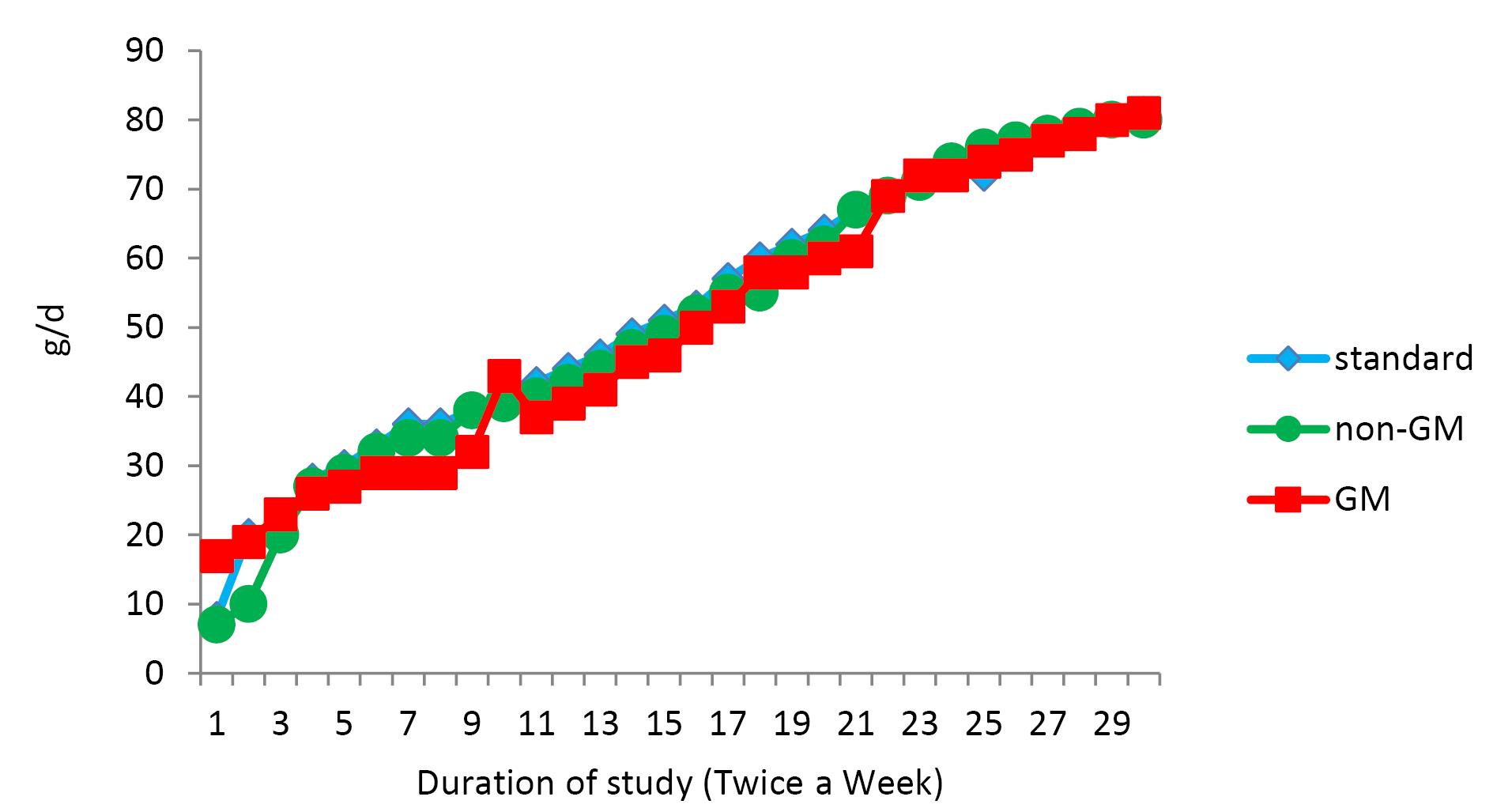
Figure 2.
Food Intake Curves Based on Twice a Week Measurements During the Study. Note. SE: Standards error; GM: Genetically modified. The curves show group means based on 6 rats /group. SE bars not shown for clarity
.
Food Intake Curves Based on Twice a Week Measurements During the Study. Note. SE: Standards error; GM: Genetically modified. The curves show group means based on 6 rats /group. SE bars not shown for clarity
Table 2.
Absolute Organ Weights in Treatment Groups
|
Organ
|
Standard
|
Non-GM
|
GM
|
P
value
|
| Spleen (g) |
2.70 ± 0.5 |
2.63 ± 0.5 |
2.83 ± 0.3 |
0.301 |
| Pancreas (g) |
3.31 ± 0.3 |
2.56 ± 0.9 |
3.05 ± 0.1 |
0.230 |
Note. GM: Genetically modified. Values are presented as mean ± SE. One-way ANOVA followed by Tukey post hoc analysis was used to compare the values between groups.
Table 3.
Hematologic Assessment in Treatment Groups
|
CBC
|
Standard
|
Non-GM
|
GM
|
Unit
|
| RBC |
0.1 ± 7.94 |
0.3 ± 7.65 |
0.2 ± 7.84 |
( × 106/µL) |
| HGB |
0.3a,b* ± 14.0 |
0.4 ± 13.41 |
0.2 ± 13.58 |
g/dL)) |
| HCT |
0.09 ± 43.38 |
2.3 ± 41.11 |
0.8 ± 42.06 |
(%) |
| MCV |
1.3 ± 53.91 |
2.9 ± 53.75 |
0.6 ± 53.98 |
FL)) |
| MCH |
0.2 ± 17.45 |
0.7 ± 17.55 |
0.3 ± 17.31 |
(PG) |
| MCHC |
0.2 ± 32.50 |
0.9 ± 32.66 |
0.2 ± 32.28 |
(g/dL) |
| RDW |
0.4 ± 14.81 |
0.4 ± 15 |
0.4 ± 14.66 |
%)) |
| HDW |
0.1 ± 2.87 |
0.2 ± 2.72 |
0.04 ± 2.95 |
(mg/dL) |
| WBC |
2.2 ± 7.96 |
2 ± 6.54 |
1.8 ± 5.88 |
( × 103/µL) |
| PLT |
129.4 ± 698 |
40.8 ± 643.83 |
52.3 ± 717 |
( × 103/µL) |
| MPV |
0.1 ± 4.46 |
0.2 ± 4.75 |
0.1 ± 4.65 |
(FL) |
| PDW |
0.8 ± 57.13 |
0.9 ± 57.63 |
0.5 ± 57.51 |
(%) |
| PCT |
0.05 ± 0.34 |
0.01 ± 0.33 |
0.36 ± 0.02 |
%)) |
| NEUT |
0.2 ± 0.77 |
0.5 ± 0.81 |
0.1 ± 0.51 |
( × 103/µL) |
| LYMP |
1.8 ± 5.36 |
1.7 ± 4.42 |
1.3 ± 3.96 |
( × 103/µL) |
| MONO |
0.3 ± 1.43 |
0.4 ± 1.07 |
0.4 ± 1.24 |
( × 103/µL) |
| EOS |
0.04 ± 0.06 |
0.01 ± 0.03 |
0.02 ± 0.05 |
( × 103/µL) |
| BASO |
0.01 ± 0.007 |
0.01 ± 0.007 |
0.01 ± 0.008 |
( × 103/µL) |
| LUC |
0.18 ± 0.1 |
0.21 ± 0.1 |
0.13 ± 0.8 |
( × 103/µL) |
Note. GM: Genetically modified; CBC: Complete blood cell; RBC: Red blood cell; HGB: Hemoglobin; HCT: Hematocrit; MCV: Mean corpuscular volume; MCH: Mean corpuscular hemoglobin; MCHC: Mean corpuscular hemoglobin concentration; RDW: Red cell distribution width; HDW: Hemoglobin distribution width; WBC: White blood cell; PLT: Blood platelet count; MPV: Mean platelet volume; PDW: Platelet distribution width; PCT: Total platelet mass; NEUT: Neutrophils; LYMP: Lymphocytes; MONO: Monocytes; EOS: Eosinophils; BASO: Basophils; LUC: Large unstained cells.
Values are presented as mean ± SE, and each group consists of five rats. One-way ANOVA followed by Tukey post hoc analysis was used to compare the values between groups. a a significant difference versus non-GM Group; b a significant difference versus GM group and non-GM groups (*P < 0.05).
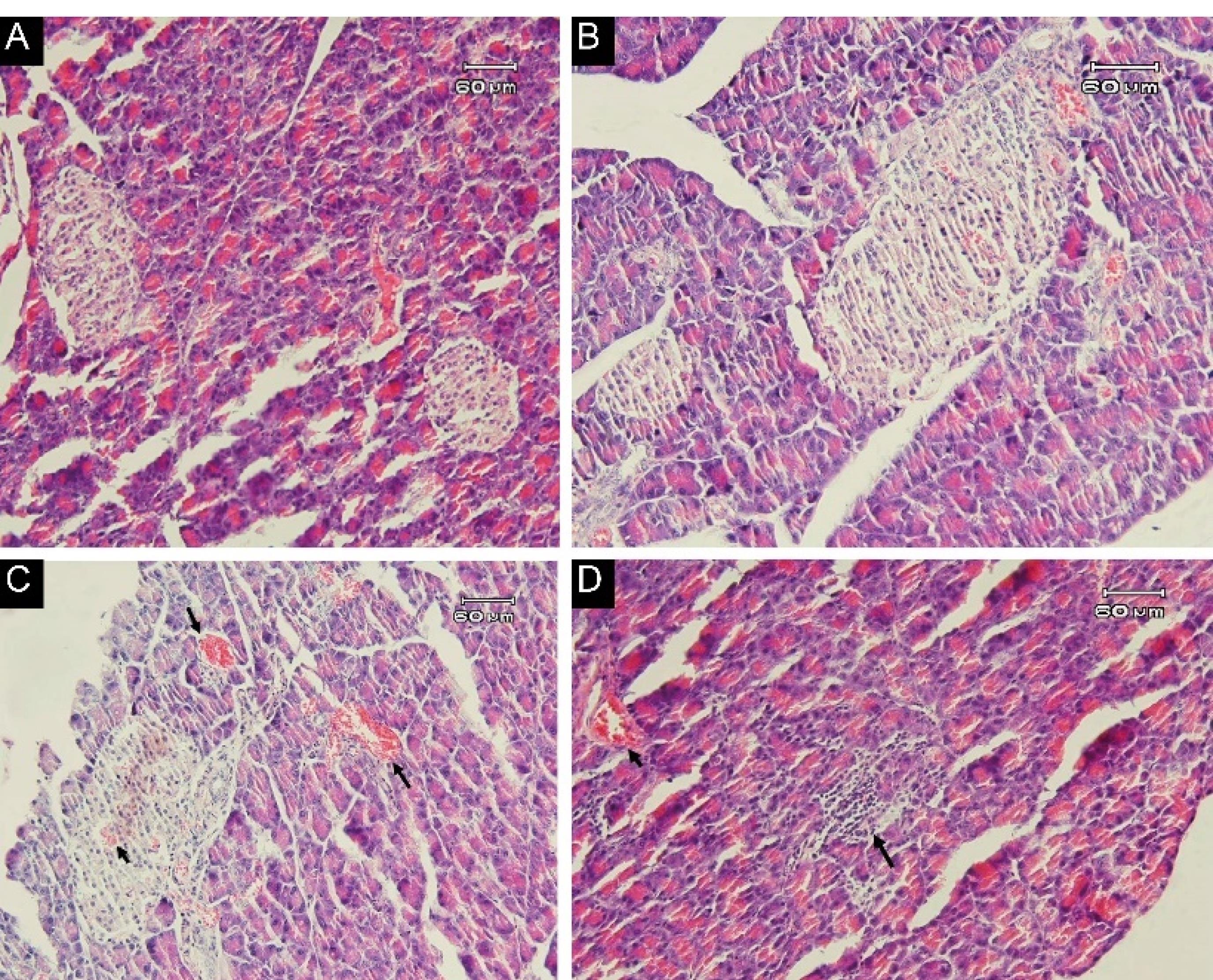
Figure 3.
Photomicrographs of Pancreas Tissue of Rats Stained with H & E: (A) standard diet, showing the normal structure of pancreas tissue. (B) Non-GM diet for 90 days, denoting the normal structure of pancreas tissue. (C) GM diet for 90 days, indicating a severe congestion in the exocrine (long arrow) and endocrine (small arrow) section. (D) GM diet for 90 days, showing, the presence of inflammatory cells in the focal region of the exocrine (long arrow) and vascular congestion (small arrow). (Magnification: 100 × ). Note. GM: Genetically modified
.
Photomicrographs of Pancreas Tissue of Rats Stained with H & E: (A) standard diet, showing the normal structure of pancreas tissue. (B) Non-GM diet for 90 days, denoting the normal structure of pancreas tissue. (C) GM diet for 90 days, indicating a severe congestion in the exocrine (long arrow) and endocrine (small arrow) section. (D) GM diet for 90 days, showing, the presence of inflammatory cells in the focal region of the exocrine (long arrow) and vascular congestion (small arrow). (Magnification: 100 × ). Note. GM: Genetically modified
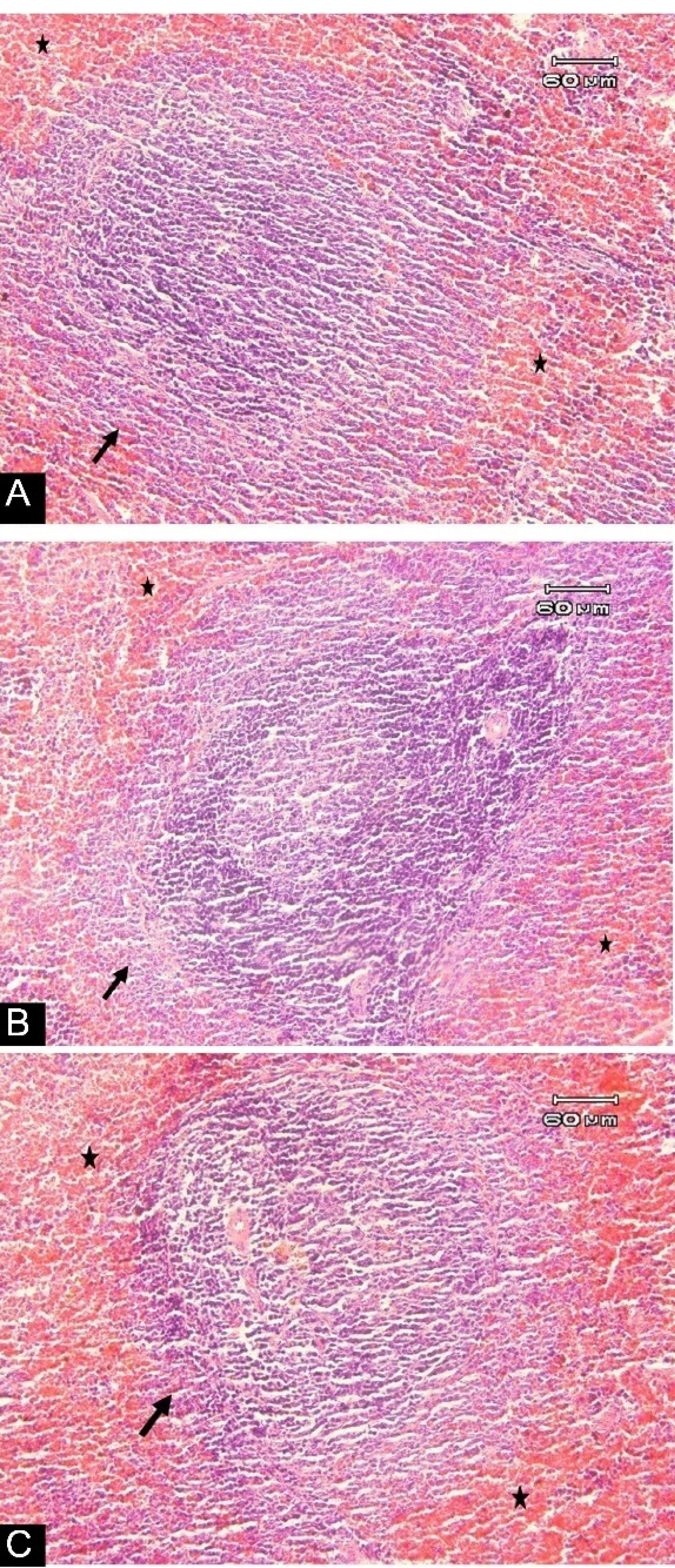
Figure 4.
Photomicrographs of the Spleen Tissue of Rats Stained with H & E: (A) Standard diet rats, showing the natural structure of lymphoid follicle (arrow), with mild congestion (stars) of spleen tissue. (B) Non-GM diet for 90 days, denoting the natural structure of lymphoid follicle (arrow), with mild congestion (stars) of spleen tissue. (C) GM diet for 90 days, displaying the natural structure of lymphoid follicle (arrow), with mild congestion (stars) of spleen tissue. (magnification: 100 × ). Note. GM: Genetically modified
.
Photomicrographs of the Spleen Tissue of Rats Stained with H & E: (A) Standard diet rats, showing the natural structure of lymphoid follicle (arrow), with mild congestion (stars) of spleen tissue. (B) Non-GM diet for 90 days, denoting the natural structure of lymphoid follicle (arrow), with mild congestion (stars) of spleen tissue. (C) GM diet for 90 days, displaying the natural structure of lymphoid follicle (arrow), with mild congestion (stars) of spleen tissue. (magnification: 100 × ). Note. GM: Genetically modified
Discussion
The insecticidal features of Bt genes have been known for over a century, and insecticidal cry proteins made by Bt have been exerted as expandable products on a remarkable scale since the early 1970s.24 Bt genes are now becoming ubiquitous in agriculture for effective pest control via recombinant DNA technology 25. However, there are several differences between the possible toxicity or non-toxicity of these products. Animal models generate precious data about the health hazards of GM plants for both livestock and human consumption.11,26,27 The experimental materials used in this work include a laboratory diet containing 10% GM soybean oil. The present study examined the possible toxic effects of a GM soybean oil diet on rats. Accordingly, the hematological values for the three groups were similar with a slight increase in hemoglobin concentrations in the standard group versus the two other groups. Generally, it was demonstrated that hemoglobin concentration is reduced due to anemia as also confirmed by previous studies25,28,29 possibly because of the presence of Bt toxin in food that causes RBCs to degrade.30 Similar to our findings, a study indicated no differences in the hematological values after 90-day feeding with Bt corn in broiler chicks.31 Likewise, no significant difference was found in hematological values in a 90-day animal study with a GM-rice diet.23
Histopathology examinations in the current study revealed changes in the pancreas tissues of animals fed with a diet containing GM soybean oil, while no changes were observed in those of other two groups. Moreover, significant modifications were reported in the pancreatic tissue of rats fed with GM soybean, including changes in chromatin and an increase in nuclear fibers.32 Another study revealed changes in pancreatic weight of Wistar rats fed GM rice for 90 days.33 Pancreatic damage is a result of complex pathophysiologic processes due to the transmission of bacteria, endotoxemia, vascular hypertrophy, ischemia, vascular injury, and thrombosis.27,34 It has also been suggested that the Cry1AB protein induces digestive tract damage and the infiltration of bacteria and germs into internal organs, infecting the abdominal cavity and other tissues, including the pancreas.35
On the other hand, no histologic changes were reported in spleen tissue except for minor congestion in all three groups, generally occurring due to anesthesia in rats. Several studies reported no change in the spleen tissue of GM-fed animals. In a study by Lin et al,36 after feeding GM virus-resistant papaya fruit for 90 days in rats, no changes were observed in the spleen tissue. Similar results were also reported after GM-maize feeding.2 Several other studies also reported similar findings with no change in the spleen tissue of GM-fed animals.37-39 The possible reason is that immediate allergic induction in animals is extremely low.40 These valuable findings indicate that other animal studies confirming these results would elucidate the social and practical implications of GM foods. This issue will be directed towards the widespread consumption of GM foods in society by humans. However, the present study had some limitations, too. It seems that direct access to transgenic plants and their oil would provide better clarification about its effect. Furthermore, it seems that it is better to evaluate the long-term effects of GM foods; nevertheless, the current study just assessed the short-term effects of GM feeding.
Conclusion
The results of this study indicated that a diet containing transgenic products causes changes in several vital organs, including pancreas tissues. Although these changes seem minor at first, they may cause serious damage over time. Further experimental studies on different animal species and more GM food products may warrant more clarification.
Ethics statement
All experiment process was conducted following the ethical guidelines of the National Institutes of Health (NIH) for the care and use of laboratory animals (NIH; Publication No. 85-23, revised 1985) and was approved by the Veterinary Ethics Committee of the Tabriz University of Medical Sciences (Code: IR.TBZMED.VCR.REC.1397.190).
Disclosure of funding source
The current work was financially supported by a grant from Research Undersecretary of Tabriz University of Medical Sciences (Code: IR.TBZMED.VCR.REC.1397.190).
Conflict of interests declaration
The authors declare no conflict of interests.
Author contributions
Conceptualization: Mahdieh Abbasalizad-Farhangi, Mehran Mesgari-Abbasi.
Data curation: Horyie Taheri, Mehran Mesgari-Abbasi, Mehran Mesgari-Abbasi.
Formal analysis: Horyie Taheri.
Funding acquisition: Mahdieh Abbasalizad-Farhangi, Horyie Taheri, Mehran Mesgari-Abbasi.
Investigation: Mahdieh Abbasalizad-Farhangi, Mehran Mesgari-Abbasi.
Methodology: Mahdieh Abbasalizad-Farhangi.
Project administration: Mahdieh Abbasalizad-Farhangi, Mehran Mesgari-Abbasi.
Resources: Horyie Taheri.
Software: Horyie Taheri.
Supervision: Mahdieh Abbasalizad-Farhangi.
Validation: Mahdieh Abbasalizad-Farhangi.
Visualization: Mahdieh Abbasalizad-Farhangi.
Writing–original draft: Horyie Taheri.
Writing–review & editing: Mahdieh Abbasalizad-Farhangi.
References
- Kiliç A, Akay MT. A three generation study with genetically modified Bt corn in rats: biochemical and histopathological investigation. Food Chem Toxicol 2008; 46(3):1164-70. doi: 10.1016/j.fct.2007.11.016 [Crossref] [ Google Scholar]
- El-Shamei ZS, Gab-Alla AA, Shatta AA, Moussa EA, Rayan AM. Histopathological changes in some organs of male rats fed on genetically modified corn (Ajeeb YG). J Am Sci 2012; 8(10):684-96. [ Google Scholar]
- Costa-Font M, Gil JM, Traill WB. Consumer acceptance, valuation of and attitudes towards genetically modified food: review and implications for food policy. Food Policy 2008; 33(2):99-111. doi: 10.1016/j.foodpol.2007.07.002 [Crossref] [ Google Scholar]
- Fortin DR, Renton MS. Consumer acceptance of genetically modified foods in New Zealand. Br Food J 2003; 105(1/2):42-58. doi: 10.1108/00070700310467483 [Crossref] [ Google Scholar]
- Tudisco R, Lombardi P, Bovera F, dˇAngelo D, Cutrignelli MI, Mastellone V. Genetically modified soya bean in rabbit feeding: detection of DNA fragments and evaluation of metabolic effects by enzymatic analysis. Anim Sci 2006; 82(2):193-9. doi: 10.1079/asc200530 [Crossref] [ Google Scholar]
- Ewen SW, Pusztai A. Effect of diets containing genetically modified potatoes expressing Galanthus nivalis lectin on rat small intestine. Lancet 1999; 354(9187):1353-4. doi: 10.1016/s0140-6736(98)05860-7 [Crossref] [ Google Scholar]
- Vecchio L, Cisterna B, Malatesta M, Martin TE, Biggiogera M. Ultrastructural analysis of testes from mice fed on genetically modified soybean. Eur J Histochem 2004; 48(4):448-54. [ Google Scholar]
- Trabalza-Marinucci M, Brandi G, Rondini C, Avellini L, Giammarini C, Costarelli S. A three-year longitudinal study on the effects of a diet containing genetically modified Bt176 maize on the health status and performance of sheep. Livest Sci 2008; 113(2-3):178-90. doi: 10.1016/j.livsci.2007.03.009 [Crossref] [ Google Scholar]
- Dona A, Arvanitoyannis IS. Health risks of genetically modified foods. Crit Rev Food Sci Nutr 2009; 49(2):164-75. doi: 10.1080/10408390701855993 [Crossref] [ Google Scholar]
- Bawa AS, Anilakumar KR. Genetically modified foods: safety, risks and public concerns-a review. J Food Sci Technol 2013; 50(6):1035-46. doi: 10.1007/s13197-012-0899-1 [Crossref] [ Google Scholar]
- Oraby H, Kandil M, Shaffie N, Ghaly I. Biological impact of feeding rats with a genetically modified-based diet. Turk J Biol 2015; 39(2):265-75. doi: 10.3906/biy-1406-61 [Crossref] [ Google Scholar]
- Shirdeli M, Orlov YL, Eslami G, Hajimohammadi B, Tabikhanova LE, Ehrampoush MH. Testing safety of genetically modified products of rice: case study on Sprague-Dawley rats. Russ J Genet 2019; 55(8):962-8. doi: 10.1134/s1022795419080131 [Crossref] [ Google Scholar]
- Zhang C, Wohlhueter R, Zhang H. Genetically modified foods: a critical review of their promise and problems. Food Sci Hum Wellness 2016; 5(3):116-23. doi: 10.1016/j.fshw.2016.04.002 [Crossref] [ Google Scholar]
- Séralini G-E, Mesnage R, Clair E, Gress S, de Vendômois JS, Cellier D. Genetically modified crops safety assessments: present limits and possible improvements. Environ Sci Eur 2011; 23(1):10. doi: 10.1186/2190-4715-23-10 [Crossref] [ Google Scholar]
- Lin HY, Liao JW, Chen RS, Chang CH, Chang HW, Chang SC. Food safety assessment of commercial genetically modified soybeans in rats. Foods 2022; 11(4):496. doi: 10.3390/foods11040496 [Crossref] [ Google Scholar]
- Séralini GE, Cellier D, de Vendomois JS. New analysis of a rat feeding study with a genetically modified maize reveals signs of hepatorenal toxicity. Arch Environ Contam Toxicol 2007; 52(4):596-602. doi: 10.1007/s00244-006-0149-5 [Crossref] [ Google Scholar]
- Hemre GI, Sanden M, Bakke‐Mckellep AM, Sagstad A, Krogdahl Å. Growth, feed utilization and health of Atlantic salmon Salmo salar L fed genetically modified compared to non-modified commercial hybrid soybeans. Aquac Nutr 2005; 11(3):157-67. doi: 10.1111/j.1365-2095.2005.00328.x [Crossref] [ Google Scholar]
- Ahrorovna KD. Effect of a genetically modified product on the morphological parameters of the rat’s spleen and thymus. Eur J Mol Clin Med 2020; 7(1):3364-70. [ Google Scholar]
- Key S, Ma JK, Drake PM. Genetically modified plants and human health. J R Soc Med 2008; 101(6):290-8. doi: 10.1258/jrsm.2008.070372 [Crossref] [ Google Scholar]
- Malatesta M, Boraldi F, Annovi G, Baldelli B, Battistelli S, Biggiogera M. A long-term study on female mice fed on a genetically modified soybean: effects on liver ageing. Histochem Cell Biol 2008; 130(5):967-77. doi: 10.1007/s00418-008-0476-x [Crossref] [ Google Scholar]
- Bawa AS, Anilakumar KR. Genetically modified foods: safety, risks and public concerns-a review. J Food Sci Technol 2013; 50(6):1035-46. doi: 10.1007/s13197-012-0899-1 [Crossref] [ Google Scholar]
- Goldstein DA, Tinland B, Gilbertson LA, Staub JM, Bannon GA, Goodman RE. Human safety and genetically modified plants: a review of antibiotic resistance markers and future transformation selection technologies. J Appl Microbiol 2005; 99(1):7-23. doi: 10.1111/j.1365-2672.2005.02595.x [Crossref] [ Google Scholar]
- Schrøder M, Poulsen M, Wilcks A, Kroghsbo S, Miller A, Frenzel T. A 90-day safety study of genetically modified rice expressing Cry1Ab protein (Bacillus thuringiensis toxin) in Wistar rats. Food Chem Toxicol 2007; 45(3):339-49. doi: 10.1016/j.fct.2006.09.001 [Crossref] [ Google Scholar]
- Gajendra Babu B, Udayasuriyan V, Asia Mariam M, Sivakumar NC, Bharathi M, Balasubramanian G. Comparative toxicity of Cry1Ac and Cry2Aa δ-endotoxins of Bacillus thuringiensis against Helicoverpa armigera (H). Crop Prot 2002; 21(9):817-22. doi: 10.1016/s0261-2194(02)00044-3 [Crossref] [ Google Scholar]
- Song H, He X, Zou S, Zhang T, Luo Y, Huang K. A 90-day subchronic feeding study of genetically modified rice expressing Cry1Ab protein in Sprague-Dawley rats. Transgenic Res 2015; 24(2):295-308. doi: 10.1007/s11248-014-9844-6 [Crossref] [ Google Scholar]
- EFSA Panel on Genetically Modified Organisms (GMO). Guidance on the environmental risk assessment of genetically modified animals. EFSA J. 2013;11(5):3200. 10.2903/j.efsa.2013.3200.
- Bertoni G, Marsan PA. Safety risks for animals fed genetic modified (GM) plants. Vet Res Commun 2005; 29 Suppl 2:13-8. doi: 10.1007/s11259-005-0004-6 [Crossref] [ Google Scholar]
- Magaña-Gómez JA, de la Barca AM. Risk assessment of genetically modified crops for nutrition and health. Nutr Rev 2009; 67(1):1-16. doi: 10.1111/j.1753-4887.2008.00130.x [Crossref] [ Google Scholar]
- Monien BH, Sachse B, Meinl W, Abraham K, Lampen A, Glatt H. Hemoglobin adducts of furfuryl alcohol in genetically modified mouse models: role of endogenous sulfotransferases 1a1 and 1d1 and transgenic human sulfotransferases 1A1/1A2. Toxicol Lett 2018; 295:173-8. doi: 10.1016/j.toxlet.2018.06.008 [Crossref] [ Google Scholar]
- Then C, Bauer-Panskus A. Possible health impacts of Bt toxins and residues from spraying with complementary herbicides in genetically engineered soybeans and risk assessment as performed by the European Food Safety Authority EFSA [published correction appears in Environ Sci Eur 2017;29(1):8]. Environ Sci Eur 2017; 29(1):1. doi: 10.1186/s12302-016-0099-0 [Crossref] [ Google Scholar]
- Yonemochi C, Fujisaki H, Harada C, Kusama T, Hanazumi M. Evaluation of transgenic event CBH 351 (StarLink) corn in broiler chicks. Anim Sci J 2002; 73(3):221-8. doi: 10.1046/j.1344-3941.2002.00031.x [Crossref] [ Google Scholar]
- Malatesta M, Biggiogera M, Manuali E, Rocchi MB, Baldelli B, Gazzanelli G. Fine structural analyses of pancreatic acinar cell nuclei from mice fed on genetically modified soybean. Eur J Histochem 2003; 47(4):385-8. [ Google Scholar]
- Poulsen M, Kroghsbo S, Schrøder M, Wilcks A, Jacobsen H, Miller A. A 90-day safety study in Wistar rats fed genetically modified rice expressing snowdrop lectin Galanthus nivalis (GNA). Food Chem Toxicol 2007; 45(3):350-63. doi: 10.1016/j.fct.2006.09.002 [Crossref] [ Google Scholar]
- Zhong J, Xie Y, Huang L, Chen G, Liao H, Dang Y. Histological alteration of pancreas in rats with sepsis. Int J Clin Exp Pathol 2017; 10(5):5743-50. [ Google Scholar]
- Spök A, Eckerstorfer M, Heissenberger A, Gaugitsch H. Risk Assessment of “Stacked Events”. Vienna: Austrian Ministry for Health; 2016.
- Lin HT, Lee WC, Tsai YT, Wu JH, Yen GC, Yeh SD. Subchronic immunotoxicity assessment of genetically modified virus-resistant papaya in rats. J Agric Food Chem 2016; 64(29):5935-40. doi: 10.1021/acs.jafc.6b02242 [Crossref] [ Google Scholar]
- Chen ZL, Gu H, Li Y, Su Y, Wu P, Jiang Z. Safety assessment for genetically modified sweet pepper and tomato. Toxicology 2003; 188(2-3):297-307. doi: 10.1016/s0300-483x(03)00111-2 [Crossref] [ Google Scholar]
- de Vendômois JS, Roullier F, Cellier D, Séralini GE. A comparison of the effects of three GM corn varieties on mammalian health. Int J Biol Sci 2009; 5(7):706-26. doi: 10.7150/ijbs.5.706 [Crossref] [ Google Scholar]
- Hammond B, Dudek R, Lemen J, Nemeth M. Results of a 13 week safety assurance study with rats fed grain from glyphosate tolerant corn. Food Chem Toxicol 2004; 42(6):1003-14. doi: 10.1016/j.fct.2004.02.013 [Crossref] [ Google Scholar]
- Teshima R, Watanabe T, Okunuki H, Isuzugawa K, Akiyama H, Onodera H. Effect of subchronic feeding of genetically modified corn (CBH351) on immune system in BN rats and B10A mice. Shokuhin Eiseigaku Zasshi 2002; 43(5):273-9. doi: 10.3358/shokueishi.43.273 [Crossref] [ Google Scholar]