Int J Drug Res Clin. 2023;1:e8.
doi: 10.34172/ijdrc.2023.e8
Original Article
Silencing Interleukin-6 and Glycoprotein 130 Suppresses Growth and Induces Apoptosis in Cancer Cells
Parastoo Khodayari 1, 2  , Atefeh Khodakarami 3, Hadi Hassannia 4, Ghasem Ghalamfarsa 5, Mohammad Hojjat-Farsangi 6, Vida Hashemi 7, Farhad Jadidi-Niaragh 1, 3, 8, *
, Atefeh Khodakarami 3, Hadi Hassannia 4, Ghasem Ghalamfarsa 5, Mohammad Hojjat-Farsangi 6, Vida Hashemi 7, Farhad Jadidi-Niaragh 1, 3, 8, * 
Author information:
1Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran
3Research Center for Integrative Medicine in Aging, Aging Research Institute, Tabriz University of Medical Sciences, Tabriz, Iran
4Immunogenetic Research Center, Faculty of Medicine and Amol Faculty of Paramedical Sciences, Mazandaran University of Medical Sciences, Sari, Iran
5Cellular and Molecular Research Center, Yasuj University of Medical Sciences, Yasuj, Iran
6Bioclinicum, Department of Oncology-Pathology, Karolinska Institute, Stockholm, Sweden
7Department of Basic Science, Faculty of Medicine, Maragheh University of Medical Sciences, Maragheh, Iran
8Department of Immunology, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Background:
Various signaling pathways promote cancer growth and inhibit apoptosis in cancerous cells. The increased levels of interleukin (IL)-6 cytokine and its signaling by IL-6 receptors have been reported in various cancers, which were associated with poor prognosis. On the other hand, targeting IL-6 and IL-6 receptors was associated with ameliorating effects in cancers. Therefore, this study sought to inhibit both IL-6 and its receptor (glycoprotein 130, [gp130]) and to synergistically reduce cancer progression in vitro.
Methods:
Accordingly, 4T1 and CT26 cancer cell lines were used to analyze the efficacy of the simultaneous blockade of IL-6 and gp130. In addition, small interfering ribonucleic acid (siRNA) molecules were employed to suppress the expression of these factors. The expression of target genes was investigated using the quantitative real-time polymerase chain reaction assay. Further, an MTT assay was applied to study cell survival. Finally, cytokine was measured by enzyme-linked immunosorbent assay.
Results:
The transfection of cancer cells by lipofectamine led to significant downregulation of these factors in both cell lines. Moreover, the downregulation of IL-6 and gp130 caused significant cell death induction, which was associated with the reduced proliferative potential of both cells. Eventually, IL-6 silencing markedly suppressed the secretion of IL-6 in both cells.
Conclusion:
These findings imply that the simultaneous silencing of IL-6 and gp130 can be considered a potential anticancer therapeutic approach that should be further considered in future studies.
Keywords: Interleukin 6, Cancer immunotherapy, Glycoprotein 130, siRNA, Interleukin-6 receptor
Introduction
Various factors in the tumor microenvironment help cancer growth and suppress anti-tumor immune responses.1,2 One of the main anti-cancer immunotherapeutic approaches is the modulation of the tumor microenvironment to limit the expansion of cancer cells and provide an optimum condition for the action of anti-tumor immune responses.3,4 Therefore, the blockade of the main contributing factors to cancer growth and immune suppression in the tumor site is considered an efficient treatment strategy.
Several studies have shown that the secretion of interleukin (IL)-6 is meaningfully upregulated in the tumor region.5 Both cancer and nan-cancerous stromal cells are involved in the generation of IL-6. Among the normal cells, both immune and non-immune cells such as monocytes, lymphocytes, fibroblasts, keratinocytes, and adipocytes can generate and secrete IL-6.6 Moreover, various solid and hematopoietic cancers can produce this cytokine.7 Interestingly, the upregulation of IL-6 was associated with cancer progression and limited responses to conventional anti-cancer therapies such as chemotherapy. On the other hand, the upregulation of IL-6 in the tumor microenvironment was accompanied by the upregulation of downstream signaling factors participating in the cancer growth.8 The induction of these signaling pathways is due to IL-6 receptor activation. The expression of the IL-6 receptor is detected in both immune and non-immune cancer cells. The cell membrane expressed IL-6 receptor has a short cytoplasmic domain that cannot drive IL-6 signaling. Therefore, it uses the help of another transmembrane protein called gp130,9 which is also expressed on various cells, and in combination with IL-6R, it contributes to tissue homeostasis, cell survival, growth, and development. IL-6R signaling leads to the induction of Janus kinase (JAK) tyrosine kinases and the phosphorylation of the signal transducer and activator of transcription 3 (STAT3). The IL-6/JAK/STAT3 axis is critical in various key characteristics of tumor cells, including transformation, survival, angiogenesis, metastasis, and proliferation.10,11
As mentioned before, several cells, including cancer and non-cancer cells, can produce IL-6 in the tumor site.12 It is demonstrated that cancer cells consume IL-6 in an autocrine manner and, to a lower extent, in a paracrine manner.13 Regarding the importance of the IL-6/IL-6R axis in cancer progression, several investigators have attempted to block this axis through various therapeutic strategies.14 The current study aimed to block the IL-6-mediated growth of cancerous cells by silencing IL-6 and gp130 in cancer cells using small interfering ribonucleic acid (siRNA) technology. It was hypothesized that the silencing of IL-6 and the main molecule in its signaling (gp130) can synergistically deprive cancer cells of IL-6-mediated cancer-promoting signaling pathways, leading to cancer progression.
Methods
Reagents and Cell Lines
Murine cell lines, including CT26 (colon cancer) and 4T1 (breast cancer), were purchased from the National Cell Bank of Iran (Pasteur Institute of Iran, Tehran, Iran). Both cell lines were cultured in RPMI-1640 medium containing 10% fetal bovine serum and supplemented with 100 mg/mL streptomycin and 100 unit/mL penicillin (which were all bought from Gibco, Grand Island, NY).
Lipofectamine 2000 was purchased from Sigma (St. Louis, MO, USA). Moreover, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) Cell Proliferation Assay Kit was obtained from Roche (Mannheim, Germany). Further, siRNA molecules against IL-6, gp130, and irrelevant control siRNA were purchased from Santa Cruz (CA, USA). Finally, the enzyme-linked immunosorbent assay (ELISA) kit for the assessment of IL-6 was purchased from eBioscience (San Diego, CA, USA).
Transfection of Cells With siRNA
To transfect cancer cells with siRNA, 1.5 × 104 4T1 and CT26 cells were cultured in 96-well plates for 24 hours. Subsequently, the cells were transfected with 60 pM of each siRNA using Lipofectamine 2000 (Sigma) according to guidelines indicated in the kit and incubated for 48 hours. Then, the cells were analyzed for gene expression.
Real-time Polymerase Chain Reaction
Gene expression was analyzed using the quantitative real-time polymerase chain reaction (qPCR).15 RNA extraction was performed by RNA extraction kits (Invitrogen, Oslo, Norway). Furthermore, complementary DNA was synthesized by using the cDNA synthesis kit (Bioneer, Daejeon, Korea).
Primer sequences for the analysis of target genes were designed based on our previous reports.16 The primer sequences are provided in Table 1.16,17 Subsequently, the SYBR® Green RT-PCR master mixture (Takara Bio Inc., Japan) and the Light-Cycler480 RT-PCR system (Roche) were applied to amplify and evaluate the expression of target genes. The standard and melting curves were created to confirm the test accuracy. The ΔΔCT method was used to analyze the data, and β-actin was considered a housekeeping gene.
Table 1.
Primer Sequences
|
Gene Name
|
Forward
|
Reverse
|
| β-actin |
5′-GGTCATCACTATTGGCAACG-3′ |
5′-ACGGATGTCAACGTCACACT-3′ |
| IL-6 |
5′- ATCCAGTTGCCTTCTTGGGACTGA -3′ |
5′- TAAGCCTCCGACTTGTGAAGTGGT -3′ |
| Gp130 |
5′- ATTTGTGTGCTGAAGGAGGC -3′ |
5′- ATTTGTGTGCTGAAGGAGGC -3′ |
| MMP9 |
5′- ACACGACATCTTCCAGTACC -3′ |
5′- CAGGAGGTCGTAGGTCACGTAGC -3 |
| MMP2 |
5′- TGTGTCTTCCCCTTCACTTT -3′ |
5′- GATCTGAGCGATGCCATCAA -3′ |
| BIM |
5′- GAGATACGGATTGCACAGGA -3′ |
5′- ATTTGAGGGTGGTCTTCAGC -3′ |
| BCL-2 |
5′- GGCTGGGGATGACTTCTCTC -3′ |
5′- ACAATCCTCCCCCAGTTCAC - 3′ |
| VEGF |
5′ - CTGGATATGTTTGACTGCTGTGGA-3′ |
5′ - GTTTCTGGAAGTGAGCCAATGTG-3′ |
| FGF |
5′- CCCACCAGGCCACTTCAA -3′ |
5′- GATGGATGCGCAGGAAGAA -3′ |
Note. IL: Interleukin; Gp: Glycoprotein; MMP: Matrix metallopeptidase-9; BCL-2: B cell lymphoma/leukemia gene-2; VEGF: Vascular endothelial growth factor; FGF: Fibroblast growth factor.
Cytotoxicity Assay
MTT assay was employed to study the effect of siRNA transfection on the viability of cancer cell lines. Accordingly, cancer cells (1.5 × 104 cells) were seeded in 96-well plates for 24 hours and subsequently transfected with siRNA molecules. Untreated cells and DMSO (0.2%) were considered as the negative and positive control, respectively. After 24 or 48 hours of incubation, the supernatant of cells was changed by using 100 μL of a medium containing the MTT solution (10 μL) and incubated for 4 hours. Finally, DMSO (100 μL) was added to each well for 4 hours, and the absorbance was assessed at 490 nm.18
Enzyme-Linked Immunosorbent Assay
The ELISA assay was used to study the impact of siRNA transfection on the secretion of IL-6. In brief, CT26 and 4T1 cells (1.5 × 104 cells) were seeded in 96-well plates for 24 hours. Afterward, the cells were transfected with siRNA molecules for 48 hours. The supernatant of the cells was then collected and utilized to analyze the concentration of IL-6 using the ELISA kit (eBioscience).19
Proliferation Assay
Bromodeoxyuridine (BrdU) incorporation kit was used to assess the impact of the siRNA transfection related to the proliferative potential of cancer cells. Briefly, 1.5 × 104 cells were cultured in 96-well plates for 24 hours and then transfected with siRNA molecules for 48 hours. Next, cancer cells were tagged with BrdU for 2 hours and incubated with the anti-BrdU monoclonal antibody for 1 hour. The Horseradish peroxidase-conjugated goat anti-mouse antibody and the peroxidase substrate were then added to the cultured cells. The percent of BrdU-incorporated cells was finally assessed by a microplate reader.20
Statistical Analysis
The statistical analysis was performed by the SPSS software; version 20. A two-way ANOVA test was applied to investigate the differences between groups, and P < 0.05 was considered statistically significant. All tests were performed in triplicate and repeated at least twice.
Results
The Use of siRNAs Meaningfully Downregulated IL-6 and gp130 in Cancer Cell Lines
The results demonstrated that anti-IL-6 specific siRNA could markedly suppress the mRNA expression of this factor in both cell lines, while it had also minor effects on gp130 expression. Moreover, the simultaneous transfection of cells by both siRNAs had the greatest effect on reducing IL-6 mRNA levels (Figures 1a and b).
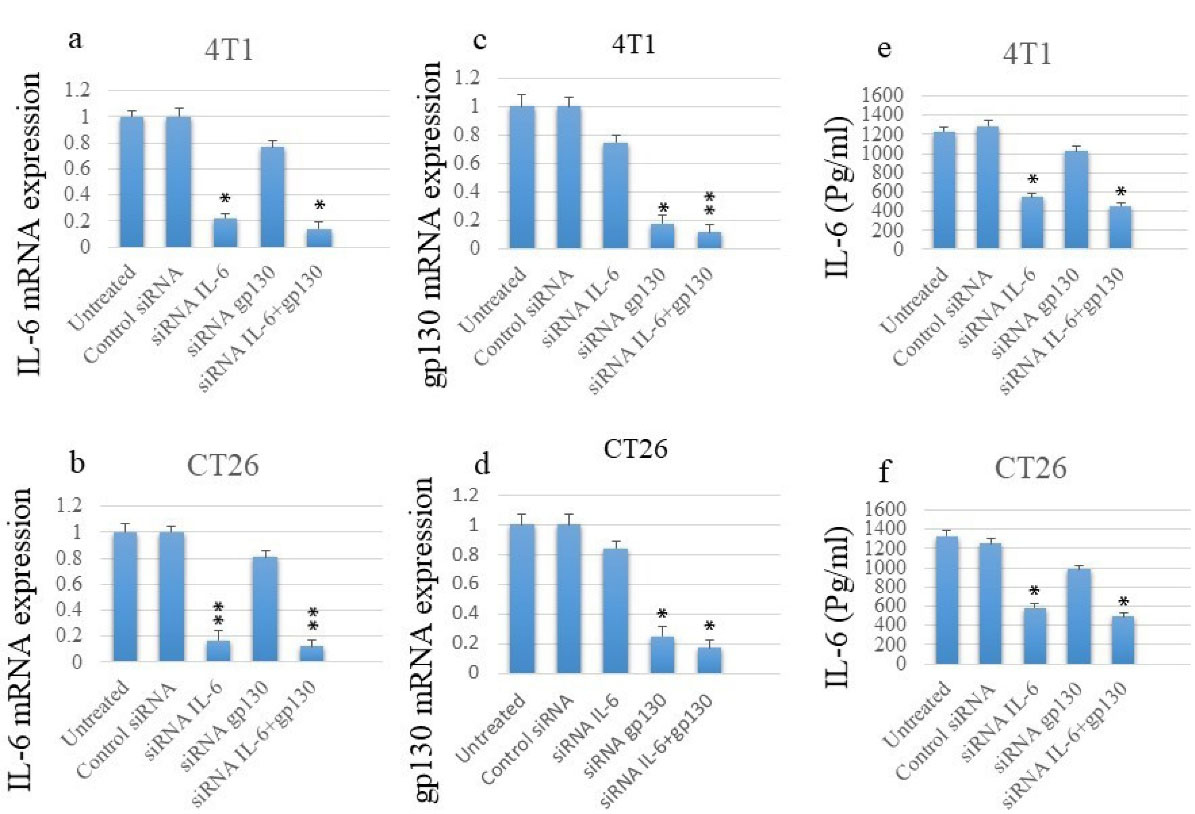
Figure 1.
Transfection of siRNA Suppresses the mRNA and Protein Expression Levels of IL-6 and mRNA Levels of gp130 in Cancer Cell Lines Based on qPCR and ELISA Assays. Note. siRNA: Small interfering ribonucleic acid; mRNA: Messenger ribonucleic acid; IL: Interleukin; gp: Glycoprotein; qPCR: Quantitative polymerase chain reaction; ELISA: Enzyme-linked immunosorbent assay
.
Transfection of siRNA Suppresses the mRNA and Protein Expression Levels of IL-6 and mRNA Levels of gp130 in Cancer Cell Lines Based on qPCR and ELISA Assays. Note. siRNA: Small interfering ribonucleic acid; mRNA: Messenger ribonucleic acid; IL: Interleukin; gp: Glycoprotein; qPCR: Quantitative polymerase chain reaction; ELISA: Enzyme-linked immunosorbent assay
Similar results were observed regarding the expression of gp130. Although anti-gp130 siRNA significantly suppressed gp130 expression, it slightly reduced IL-6 expression. Additionally, the co-transfection of cells by both siRNAs had the greatest impact on gp130 mRNA expression (Figures 1c and d).
To further analyze the impact of siRNA on the IL-6, IL-6 protein levels in the supernatant of cultured cell lines were studied using ELISA assay. Similar to the mRNA results, siRNA transfection meaningfully suppressed IL-6 secretion, and co-transfection had the greatest effect on IL-6 secretion levels (Figures 1e and f).
Silencing IL-6 and gp130 Meaningfully Induced Cell Death in Cancer Cells
Cancer cells were incubated with anti-IL-6 and anti-gp130 siRNAs, and the viability of cancer cells was measured after 24 and 48 hours. The results revealed that the silencing of either IL-6 or gp130 could markedly induce cell death in cancer cells over time. Interestingly, co-silencing of IL-6 and gp130 could additively increase cell death in both cells (Figures 2a and b).

Figure 2.
Silencing IL-6 and gp130 in Cancer Cells Induces Cell Death Associated With the Upregulation of BIM and Downregulation of Bcl-2. Note. IL: Interleukin; gp: Glycoprotein
.
Silencing IL-6 and gp130 in Cancer Cells Induces Cell Death Associated With the Upregulation of BIM and Downregulation of Bcl-2. Note. IL: Interleukin; gp: Glycoprotein
The expression of apoptosis-involved factors, including BIM and Bcl-2, was evaluated to study the mechanism by which silencing IL-6 and gp130 induced cell death. As shown in Figures 2c and d, while the silencing of either IL-6 or gp130 could considerably reduce the expression of the anti-apoptotic Bcl-2 factor, their simultaneous silencing had the greatest impact on the downregulation of Bcl-2 in both cells. Further, the treatment of cell lines with siRNAs could significantly upregulate the pro-apoptotic BIM factor in both cell lines (Figures 2e and f).
Downregulation of IL-6 and gp130 Inhibited Proliferation of Cancer Cells
The proliferative potential of cancerous cells following exposure to anti-IL-6 and gp130 siRNA molecules was investigated using the ELISA-based BrdU incorporation method. As depicted in Figures 3a and b, the exposure of cancer cell lines to either of IL-6- or gp130-specific siRNAs could potentially inhibit the proliferation of both cancer cell lines. Moreover, the co-transfection of cancer cells with both siRNA molecules had the highest impact on the inhibition of expansion.
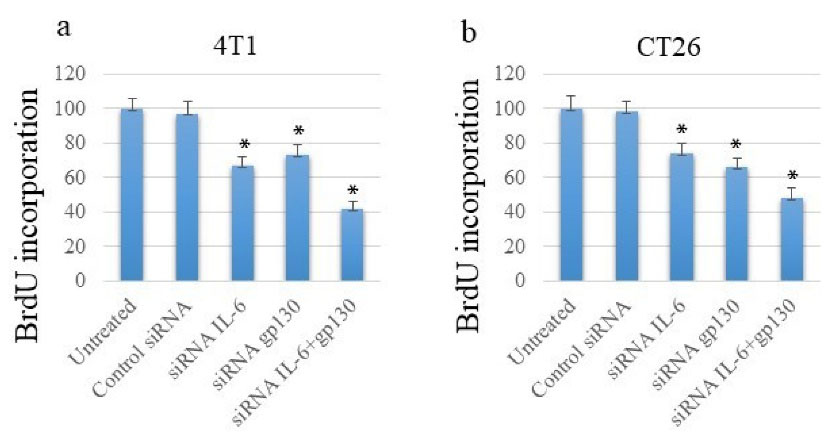
Figure 3.
Co-silencing IL-6 and gp130 Suppresses Cancer Cell Proliferation as Assessed by BrdU Incorporation Assay. Note. IL: Interleukin; gp: Glycoprotein; BrdU: Bromodeoxyuridine
.
Co-silencing IL-6 and gp130 Suppresses Cancer Cell Proliferation as Assessed by BrdU Incorporation Assay. Note. IL: Interleukin; gp: Glycoprotein; BrdU: Bromodeoxyuridine
Simultaneous Blockade of IL-6 and gp130 Reduced the Angiogenic and Metastatic Potential of Cancer Cells
The results showed that the silencing of either IL-6 or gp130 significantly reduced the expression of vascular endothelial growth factor (VEGF) in both cell lines; however, simultaneous silencing by two siRNAs had the greatest effect on the expression of VEGF (Figures 4a and b).
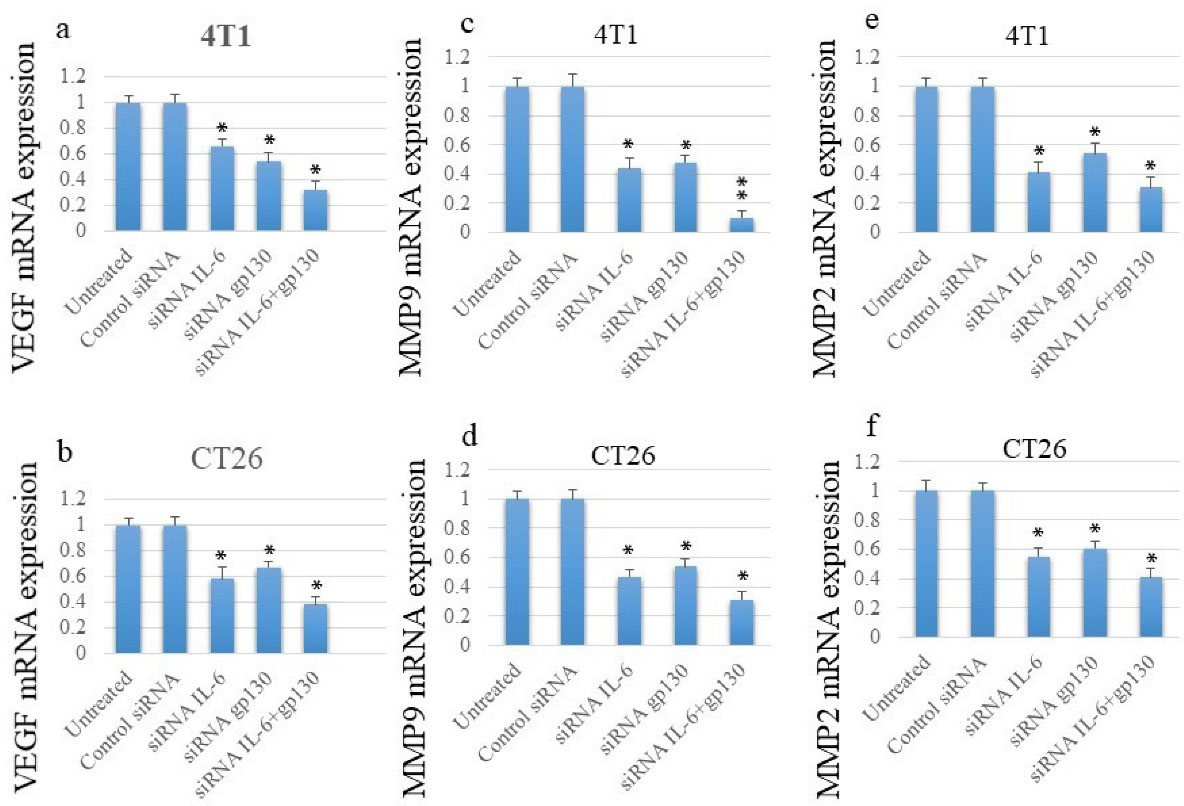
Figure 4.
The siRNA Transfection Inhibited the Expression of Genes Involved in the Angiogenesis and the Metastasis Process in Cancer Cell Lines. Note. siRNA: Small interfering ribonucleic acid
.
The siRNA Transfection Inhibited the Expression of Genes Involved in the Angiogenesis and the Metastasis Process in Cancer Cell Lines. Note. siRNA: Small interfering ribonucleic acid
Similar to VEGF, the transfection of cancer cells with anti-IL-6 or -gp130 siRNAs could markedly inhibit the expression of matrix metallopeptidase-2 (MMP2) and matrix metallopeptidase-9 (MMP9); conversely, the greatest effect was observed following the simultaneous silencing of IL-6 and gp130 (Figure 4c-f).
Discussion
The overexpression of cancer-promoting factors in the tumor microenvironment is one of the main obstacles in various anti-cancer therapeutic approaches. Therefore, targeting these factors is considered a potential therapeutic approach for various cancers.21
The IL-6/JAK/STAT3 signaling pathway is one of the main tumor-promoting axes involved in the growth and development of cancers. Several investigators have attempted to block this signaling pathway through the blockade of its main components, including, IL-6, IL-6R, gp130, JAK, and STAT3.22
In this study, it was sought to block this axis through the silencing of two critical components, including IL-6 and gp130. IL-6 is the first component in this axis that initiates it, while gp130 mediates and induces the JAK/STAT3 signaling pathway. Hence, this axis was blocked in two critical sites, thus its compensation may be impossible for cancer cells.
In this study, two different cancer cell lines were used to investigate the universal therapeutic potential of our therapeutic strategy. Furthermore, murine cell lines were employed to be able to make murine cancer models in future studies.
Our results represented that the silencing of IL-6 and gp130 could significantly enhance cell death in cancer cells, which was associated with the upregulation of BIM and the downregulation of Bcl-2 in cancer cell lines. Additionally, siRNA transfection significantly inhibited the proliferative potential of both cancer cell lines. The anti-angiogenic and anti-metastatic potential of this therapeutic strategy was also demonstrated after treatment. Based on the results, the silencing of IL-6 and gp130 could meaningfully inhibit the expression of the main factors involved in the angiogenesis and metastasis, including the VEGF, MMP2, and MMP9.
Several investigators attempted to block IL-6, IL-6Rs, or downstream signaling factors such as JAKs and STAT3 for cancer therapy. Siltuximab is one of these therapeutics that is anti-IL-6 chimeric mAb. Its efficacy is represented in the treatment of various cancers23-25 and approved for multicentric Castleman’s disease.26,27 Sirukumab and olokizumab are the other anti-IL-6 monoclonal antibodies, the efficacy of which is not yet evaluated in cancer patients.28 The mAb 1339 and clazakizumab are also anti-IL-6 monoclonal antibodies that have been evaluated for the treatment of various cancers.26
Some investigators sought to block IL-6/JAK/STAT3 signaling through the inhibition of IL-6R. For instance, the efficacy of Tocilizumab (a humanized anti-IL-6R monoclonal antibody) was examined in the treatment of a variety of cancers.29 Gp130 is also considered a potential target for blocking IL-6 signaling. Accordingly, soluble gp130-Fc fusion protein could efficiently suppress the trans-signaling of IL-6R.22 Anti-gp130 monoclonal antibodies are also used to directly block gp130 in preclinical studies.30 The chemical inhibitors of gp130 have been developed and evaluated in various studies.
Madindoline A and SC144 are two chemical antagonists of a gp130 that can suppress the IL-6/IL-6R signaling pathway.31,32
Raloxifene, LMT-28, and bazedoxifene are the other chemical therapeutics that can block the interaction of IL-6-gp130.33,34
The findings of this investigation imply that IL-6 blockade in cancer cells can be considered a worthy therapeutic approach for cancer therapy. There are several options to design a strategy for the blockade of IL-6-induced signaling. The blockade of IL-6, IL-6R, gp130, JAKs, or STAT3 is an important target of this pathway that is employed as the therapeutic target in different studies. The question regarding which target can be associated with better therapeutic consequences remains elusive and should be precisely considered in future studies. However, in this study, we implied that simultaneous targeting of IL-6 (as the initiator and first component of this signaling pathway) and gp130 (as the main component in driving and initiating the signaling pathway) can efficiently prevent cancer cell growth and survival. However, this study had some limitations such as the lack of study in preclinical models that should be performed in future studies. Following the assessment of the efficacy in animal models, investigating the efficacy of this strategy in cancer patients will clarify the significance of this therapeutic approach.
Ethics statement
This study was ethically approved by Tabriz University of Medical Sciences (Ethic number: IR.TBZMED.VCR.REC.1401.254).
Conflict of interests declaration
There is nothing to declare.
Acknowledgments
We would like to thank Tabriz University of Medical Sciences for the financial support of this study (grant number: 69968).
References
- Kazemi T, Younesi V, Jadidi-Niaragh F, Yousefi M. Immunotherapeutic approaches for cancer therapy: an updated review. Artif Cells Nanomed Biotechnol 2016; 44(3):769-79. doi: 10.3109/21691401.2015.1019669 [Crossref] [ Google Scholar]
- Gravitz L. Cancer immunotherapy. Nature 2013; 504(7480):S1. doi: 10.1038/504S1a [Crossref] [ Google Scholar]
- Haji-Fatahaliha M, Hosseini M, Akbarian A, Sadreddini S, Jadidi-Niaragh F, Yousefi M. CAR-modified T-cell therapy for cancer: an updated review. Artif Cells Nanomed Biotechnol 2016; 44(6):1339-49. doi: 10.3109/21691401.2015.1052465 [Crossref] [ Google Scholar]
- Couzin-Frankel J. Cancer immunotherapy. Science 2013; 342(6165):1432-3. doi: 10.1126/science.342.6165.1432 [Crossref] [ Google Scholar]
- Knüpfer H, Preiss R. Serum interleukin-6 levels in colorectal cancer patients--a summary of published results. Int J Colorectal Dis 2010; 25(2):135-40. doi: 10.1007/s00384-009-0818-8 [Crossref] [ Google Scholar]
- Huang B, Lang X, Li X. The role of IL-6/JAK2/STAT3 signaling pathway in cancers. Front Oncol 2022; 12:1023177. doi: 10.3389/fonc.2022.1023177 [Crossref] [ Google Scholar]
- Rašková M, Lacina L, Kejík Z, Venhauerová A, Skaličková M, Kolář M. The role of IL-6 in cancer cell invasiveness and metastasis-overview and therapeutic opportunities. Cells 2022; 11(22):3698. doi: 10.3390/cells11223698 [Crossref] [ Google Scholar]
- Chen J, Wei Y, Yang W, Huang Q, Chen Y, Zeng K. IL-6: the link between inflammation, immunity and breast cancer. Front Oncol 2022; 12:903800. doi: 10.3389/fonc.2022.903800 [Crossref] [ Google Scholar]
- Manore SG, Doheny DL, Wong GL, Lo HW. IL-6/JAK/STAT3 signaling in breast cancer metastasis: biology and treatment. Front Oncol 2022; 12:866014. doi: 10.3389/fonc.2022.866014 [Crossref] [ Google Scholar]
- Bravo J, Heath JK. Receptor recognition by gp130 cytokines. EMBO J 2000; 19(11):2399-411. doi: 10.1093/emboj/19.11.2399 [Crossref] [ Google Scholar]
- Chow D, He X, Snow AL, Rose-John S, Garcia KC. Structure of an extracellular gp130 cytokine receptor signaling complex. Science 2001; 291(5511):2150-5. doi: 10.1126/science.1058308 [Crossref] [ Google Scholar]
- Masjedi A, Hashemi V, Hojjat-Farsangi M, Ghalamfarsa G, Azizi G, Yousefi M. The significant role of interleukin-6 and its signaling pathway in the immunopathogenesis and treatment of breast cancer. Biomed Pharmacother 2018; 108:1415-24. doi: 10.1016/j.biopha.2018.09.177 [Crossref] [ Google Scholar]
- Fisher DT, Appenheimer MM, Evans SS. The two faces of IL-6 in the tumor microenvironment. Semin Immunol 2014; 26(1):38-47. doi: 10.1016/j.smim.2014.01.008 [Crossref] [ Google Scholar]
- Taher MY, Davies DM, Maher J. The role of the interleukin (IL)-6/IL-6 receptor axis in cancer. Biochem Soc Trans 2018; 46(6):1449-62. doi: 10.1042/bst20180136 [Crossref] [ Google Scholar]
- Jadidi-Niaragh F, Jeddi-Tehrani M, Ansaripour B, Razavi SM, Sharifian RA, Shokri F. Reduced frequency of NKT-like cells in patients with progressive chronic lymphocytic leukemia. Med Oncol 2012; 29(5):3561-9. doi: 10.1007/s12032-012-0262-4 [Crossref] [ Google Scholar]
- Nikkhoo A, Rostami N, Farhadi S, Esmaily M, Moghadaszadeh Ardebili S, Atyabi F. Codelivery of STAT3 siRNA and BV6 by carboxymethyl dextran trimethyl chitosan nanoparticles suppresses cancer cell progression. Int J Pharm 2020; 581:119236. doi: 10.1016/j.ijpharm.2020.119236 [Crossref] [ Google Scholar]
- Masjedi A, Ahmadi A, Atyabi F, Farhadi S, Irandoust M, Khazaei-Poul Y. Silencing of IL-6 and STAT3 by siRNA loaded hyaluronate-N,N,N-trimethyl chitosan nanoparticles potently reduces cancer cell progression. Int J Biol Macromol 2020; 149:487-500. doi: 10.1016/j.ijbiomac.2020.01.273 [Crossref] [ Google Scholar]
- Masjedi A, Hassannia H, Atyabi F, Rastegari A, Hojjat-Farsangi M, Namdar A. Downregulation of A2AR by siRNA loaded PEG-chitosan-lactate nanoparticles restores the T cell mediated anti-tumor responses through blockage of PKA/CREB signaling pathway. Int J Biol Macromol 2019; 133:436-45. doi: 10.1016/j.ijbiomac.2019.03.223 [Crossref] [ Google Scholar]
- Arab S, Kheshtchin N, Ajami M, Ashurpoor M, Safvati A, Namdar A. Increased efficacy of a dendritic cell-based therapeutic cancer vaccine with adenosine receptor antagonist and CD73 inhibitor. Tumour Biol 2017; 39(3):1010428317695021. doi: 10.1177/1010428317695021 [Crossref] [ Google Scholar]
- Joshi N, Hajizadeh F, Ansari Dezfouli E, Zekiy AO, Nabi Afjadi M, Mousavi SM. Silencing STAT3 enhances sensitivity of cancer cells to doxorubicin and inhibits tumor progression. Life Sci 2021; 275:119369. doi: 10.1016/j.lfs.2021.119369 [Crossref] [ Google Scholar]
- Yu Y, Cui J. Present and future of cancer immunotherapy: a tumor microenvironmental perspective. Oncol Lett 2018; 16(4):4105-13. doi: 10.3892/ol.2018.9219 [Crossref] [ Google Scholar]
- Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol 2018; 15(4):234-48. doi: 10.1038/nrclinonc.2018.8 [Crossref] [ Google Scholar]
- Dorff TB, Goldman B, Pinski JK, Mack PC, Lara PN, Jr Jr. , Van Veldhuizen PJ Jr, et al Clinical and correlative results of SWOG S0354: a phase II trial of CNTO328 (siltuximab), a monoclonal antibody against interleukin-6, in chemotherapy-pretreated patients with castration-resistant prostate cancer. Clin Cancer Res 2010; 16(11):3028-34. doi: 10.1158/1078-0432.ccr-09-3122 [Crossref] [ Google Scholar]
- Guo Y, Nemeth J, O’Brien C, Susa M, Liu X, Zhang Z. Effects of siltuximab on the IL-6-induced signaling pathway in ovarian cancer. Clin Cancer Res 2010; 16(23):5759-69. doi: 10.1158/1078-0432.ccr-10-1095 [Crossref] [ Google Scholar]
- Hudes G, Tagawa ST, Whang YE, Qi M, Qin X, Puchalski TA. A phase 1 study of a chimeric monoclonal antibody against interleukin-6, siltuximab, combined with docetaxel in patients with metastatic castration-resistant prostate cancer. Invest New Drugs 2013; 31(3):669-76. doi: 10.1007/s10637-012-9857-z [Crossref] [ Google Scholar]
- Heo TH, Wahler J, Suh N. Potential therapeutic implications of IL-6/IL-6R/gp130-targeting agents in breast cancer. Oncotarget 2016; 7(13):15460-73. doi: 10.18632/oncotarget.7102 [Crossref] [ Google Scholar]
- Uciechowski P, Dempke WCM. Interleukin-6: a masterplayer in the cytokine network. Oncology 2020; 98(3):131-7. doi: 10.1159/000505099 [Crossref] [ Google Scholar]
- Yao X, Huang J, Zhong H, Shen N, Faggioni R, Fung M. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther 2014; 141(2):125-39. doi: 10.1016/j.pharmthera.2013.09.004 [Crossref] [ Google Scholar]
- Ando K, Takahashi F, Motojima S, Nakashima K, Kaneko N, Hoshi K. Possible role for tocilizumab, an anti-interleukin-6 receptor antibody, in treating cancer cachexia. J Clin Oncol 2013; 31(6):e69-72. doi: 10.1200/jco.2012.44.2020 [Crossref] [ Google Scholar]
- Sommer J, Effenberger T, Volpi E, Waetzig GH, Bernhardt M, Suthaus J. Constitutively active mutant gp130 receptor protein from inflammatory hepatocellular adenoma is inhibited by an anti-gp130 antibody that specifically neutralizes interleukin 11 signaling. J Biol Chem 2012; 287(17):13743-51. doi: 10.1074/jbc.M111.349167 [Crossref] [ Google Scholar]
- Tran QH, Nguyen QT, Vo NQ, Mai TT, Tran TT, Tran TD. Structure-based 3D-pharmacophore modeling to discover novel interleukin 6 inhibitors: an in silico screening, molecular dynamics simulations and binding free energy calculations. PLoS One 2022; 17(4):e0266632. doi: 10.1371/journal.pone.0266632 [Crossref] [ Google Scholar]
- Hong SS, Choi JH, Lee SY, Park YH, Park KY, Lee JY. A novel small-molecule inhibitor targeting the IL-6 receptor β subunit, glycoprotein 130. J Immunol 2015; 195(1):237-45. doi: 10.4049/jimmunol.1402908 [Crossref] [ Google Scholar]
- Tian J, Chen X, Fu S, Zhang R, Pan L, Cao Y. Bazedoxifene is a novel IL-6/GP130 inhibitor for treating triple-negative breast cancer. Breast Cancer Res Treat 2019; 175(3):553-66. doi: 10.1007/s10549-019-05183-2 [Crossref] [ Google Scholar]
- Metcalfe RD, Putoczki TL, Griffin MDW. Structural understanding of interleukin 6 family cytokine signaling and targeted therapies: focus on interleukin 11. Front Immunol 2020; 11:1424. doi: 10.3389/fimmu.2020.01424 [Crossref] [ Google Scholar]