Int J Drug Res Clin. 2:e26.
doi: 10.34172/ijdrc.2024.e26
Original Article
Upregulation of PD-L1, VISTA, and CTLA-4 Gene Expression in a Melanoma Cell Line Following Chemotherapy: An In Vitro Study
Mahya Ahmadpour Youshanlui 1  , Zahra Valedkarimi 1, Hadi Nasiri 1, Sahand Eslami 1
, Zahra Valedkarimi 1, Hadi Nasiri 1, Sahand Eslami 1  , Ali Jafarizadeh 1, 2, Kimia Motlagh Asghari 1, Alireza Abdshah 3, Arezou Jafari 1, Behzad Baradaran 1, *
, Ali Jafarizadeh 1, 2, Kimia Motlagh Asghari 1, Alireza Abdshah 3, Arezou Jafari 1, Behzad Baradaran 1, * 
Author information:
1Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2Research Center for Evidence-Based Medicine, Iranian EBM Centre: A Joanna Briggs Institute Affiliated Group, Tabriz University of Medical Sciences, Tabriz, Iran
3School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
Abstract
Background:
Melanoma, an aggressive form of skin cancer, has garnered substantial research attention due to its rising incidence and limited treatment efficacy. Tumor cells often become more aggressive post-chemotherapy, evading innate and adaptive immune responses, leading to recurrence and metastasis. Identifying mechanisms of chemotherapy resistance is crucial to reduce mortality rates. Negative immune pathways such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed death-ligand 1 (PD-L1), and V-domain Ig suppressor of T cell activation (VISTA) hinder immune responses to cancer. This study aimed to uncover the dynamic alterations in the expression patterns of key immune checkpoints VISTA, PD-L1, and CTLA-4 in melanoma cancer cell line.
Methods:
In this study, the A-375 melanoma cancer cell line was cultivated using conventional cell culture techniques. The IC50 (half-maximal inhibitory concentration) and efficacy assessment of chemotherapy drugs, docetaxel and doxorubicin, were determined using the MTT test. After treatment, an analysis of the expression of PD-L1, CTLA-4, and VISTA genes in the A-375 cell line was performed using quantitative reverse transcription-polymerase chain reaction (qRT-PCR). One-way analysis of variance (ANOVA) was used for statistical analysis. All experiments were conducted in triplicate, with significance set at P<0.05. Statistical significance is denoted as follows: *P<0.05, **P<0.01, ***P<0.001, and ****P<0.0001.
Results:
Significant upregulation of VISTA expression was observed in both ADO and ADC groups. CTLA-4 expression increased significantly in the ADC group, while no significant change was observed in the ADO group. PD-L1 expression was significantly higher in both ADO and ADC groups compared to the control group.
Conclusion:
These compelling findings underscore the profound impact of chemotherapeutic agents on the heightened expression of immune checkpoint genes, shedding light on the potential modulation of crucial pathways associated with immune response regulation.
Keywords: Melanoma, Chemotherapy, Immune checkpoint, VISTA, CTLA-4, PD-L1
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
The financial support for this study was provided through a grant from Tabriz University of Medical Sciences, Tabriz, Iran (project identification number: 71486).
Please cite this article as follows: Ahmadpour Youshanlui M, Valedkarimi Z, Nasiri H, Eslami S, Jafarizadeh A, Motlagh Asghari K, et al. Upregulation of PD-L1, VISTA, and CTLA-4 gene expression in a melanoma cell line following chemotherapy: an in vitro study. Int J Drug Res Clin. 2024; 2: e26. doi: 10.34172/ijdrc.2024.e26
Introduction
Melanoma, arising from the malignant transformation of melanocytes, is a lethal form of skin cancer and a significant global public health issue.1,2 It is also recognized for imposing a considerable health burden, particularly in terms of years of life lost.3,4
While recent strides in comprehending the genetic aspects of melanoma have been made, treatment options remain limited.5 Traditionally, surgery to remove the tumor has been the primary treatment option for early-stage melanoma, later complemented by chemotherapy.6 Surgery and radiotherapy play marginal roles in metastatic melanoma, with chemotherapy reigning supreme, especially in advanced stages.7
The efficacy of commonly used anticancer therapies, including chemotherapy, has been hindered by developing resistance through intricate mechanisms.8 Chemotherapy, as a standalone treatment, lacks a significant survival benefit. However, combining chemotherapy with immunotherapy has demonstrated enhanced clinical outcomes.9,10
Over the last decade, advancements in understanding tumor-mediated immune evasion have reshaped the treatment approach for advanced melanoma, bringing immune checkpoint proteins to the forefront.10,11 These checkpoints, integral to immune evasion, are crucial defense mechanisms forming a molecular shield that aids cancer cells in evading anti-tumor immune responses.12
Among these checkpoints, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed death-ligand 1 (PD-L1), and V-domain Ig suppressor of T cell activation (VISTA) pathways emerge as key players, potentially hindering the immune system response to cancer and influencing the response of the tumor to conventional chemotherapeutic agents.13-15 Further emphasizing the complexity, when tumor-infiltrating lymphocytes recognize tumors, they trigger the secretion of interferon-gamma (IFN-γ) and pro-inflammatory cytokines. This results in the increased expression of PD-L1 in the tumor immune microenvironment, which is acknowledged as a key prognostic and predictive biomarker, adding another layer of significance to the role of immune checkpoints in melanoma.16 In melanoma, CTLA-4 is highly expressed, representing a significant restraining pathway with upregulated expression following T-cell activation. Antibodies targeting CTLA-4 emerge as the most promising immuno-stimulatory medication for treating metastatic melanoma.17 Adding to the complexity, VISTA, an understudied immune checkpoint protein, introduces another dimension to the intricate landscape. It facilitates tumor initiation and modifies the immune microenvironment in melanoma.18
Within the realm of chemotherapy, doxorubicin and docetaxel, widely used chemotherapeutic agents, exert notable effects on the immune system and immune checkpoint expression and have been associated with the regulation of immune checkpoint expression.19,20
Our study was designed to elucidate the intricate effects of these chemotherapy agents to recognize the pivotal role of doxorubicin and docetaxel in regulating immune checkpoint expression. Focusing on the A-375 melanoma cancer cell line, we aimed to investigate the dynamic alterations in the expression patterns of key immune checkpoints, including VISTA, PD-L1, and CTLA-4.
Methods
Establishment and Culture Conditions of A-375 Melanoma Cell Line
The A-375 melanoma cell line, obtained from the National Cell Bank of Iran (NCBI Code: C136),21 was initially cultured in RPMI-1640 medium enriched with 10% fetal bovine serum (FBS). The cells were then incubated under controlled conditions of 37 °C temperature, 5% CO2, and 95% humidity. Subsequently, the cells underwent a passage process. All experiments were performed during the logarithmic phase of cell growth.
Determination of IC50 Values of Chemotherapy Drug Through Colorimetric Analysis
The drugs used in this study, doxorubicin (Shafayab Gostar® Company, Tehran, Iran) and docetaxel (Nano Alvand® Company, Tehran, Iran) were purchased in the form of prepared solutions. RPMI medium was used to dilute the drugs to the concentrations used for treating the cells. To determine the IC50 values, different doses of doxorubicin and docetaxel, ranging from 0 to 150 μg/mL, were administered to the cell culture. Subsequently, the cells underwent a 24-hour incubation. After the incubation process, the supernatant culture medium was removed. Each well was treated with 50 μL of MTT solution (2 mg/mL MTT in PBS) and 100 μL of complete culture medium, followed by incubation at 4-hour intervals. After the designated incubation period, the supernatant was removed again, and each well received 200 μL of dimethyl sulfoxide (DMSO). After a 30-minute treatment with DMSO, the plate was analyzed with an ELISA reader. The alterations in the wells were measured using the colorimetry at the wavelength range of 570-630 nm. The optical density (OD) values obtained from the ELISA reader were recorded in an Excel spreadsheet for organization and analysis. GraphPad Prism software was utilized to analyze the data, where drug concentrations and corresponding OD values were imported into an XY axis. Non-linear regression analysis was conducted to generate a dose-response curve, and the IC50 values were determined using the dose-response inhibition function in GraphPad Prism, representing the concentration where 50% of the maximal response was observed.
RNA Extraction and cDNA Synthesis from Chemotherapy-Treated Cells
The TRIzol reagent (RiboEx Kit by GeneAll, South Korea) was used to extract RNA from cells treated with chemotherapeutic drugs. Subsequently, cDNA synthesis was conducted using the Biofact kit (South Korea) according to the manufacturer’s instructions. The concentration and quality of the extracted RNAs were assessed using a NanoDrop spectrophotometer, which measured absorbance at 260 nm and 280 nm (Thermo Fisher Scientific Life Sciences, USA). For cDNA synthesis, 1 μL of the isolated RNA was employed, utilizing a universal cDNA synthesis Biofact kit.
Analysis of Gene Expression Through qRT -PCR
The mRNA expression was examined using SYBR Premix Ex Taq II (TAKARA, Japan) in the Applied Biosystems StepOnePlusTM Real-Time PCR System, a state-of-the-art instrument produced by Life Technologies Corporation in Carlsbad, USA. To standardize the initial gene expressions, GAPDH was used as the internal control.
The generated cDNA was subsequently analyzed using quantitative real-time PCR (qRT-PCR) with targeted primers. These primers, crucial for the accurate study of targeted genes, were obtained from the NCBI website (http://www.ncbi.nlm.nih.gov) and custom-designed by Takapouzist Company in Tehran. Detailed information about these primers can be found in Table 1.
Table 1.
Primer Sequences
|
Gene
|
Strand
|
Sequence 5ʹ→3ʹ
|
| PD-L1 |
Forward |
TGCCGACTACAAGCGAATTACTG |
| Reverse |
CTGCTTGTCCAGATGACTTCGG |
| VISTA |
Forward |
GCGGATGGACAGCAACATT |
| Reverse |
TTGGAGAGTCAGGGACAGGG |
| CTLA-4 |
Forward |
CATGATGGGGAATGAGTTGACC |
| Reverse |
TCAGTCCTTGGATAGTGAGGTTC |
| GAPDH |
Forward |
AACATCATCCCTGCCTCTAC |
| Reverse |
CTGCTTCACCACCTTCTTG |
Statistical Analysis
In this research, the data derived from experimental observations underwent analysis using GraphPad Prism 9.5.1. To measure and identify statistical variations between the control and experimental groups, we employed one-way ANOVA, a robust statistical tool for conducting multiple group comparisons. A P value threshold of < 0.05 was considered statistically significant. Each experiment was conducted once on four groups (four replicates).
Results
IC50 Determination of Doxorubicin and Docetaxel in A-375 Cell Line
After cell culture, an MTT assay was conducted. The half-maximal inhibitory concentrations (IC50) of doxorubicin and docetaxel in the A-375 cell line, as depicted in Figure 1, were found to be 20.29 µg/mL and 16.21 µg/mL, respectively. Docetaxel has a half-life of 86 hours, while Doxorubicin has a half-life of 48 hours.
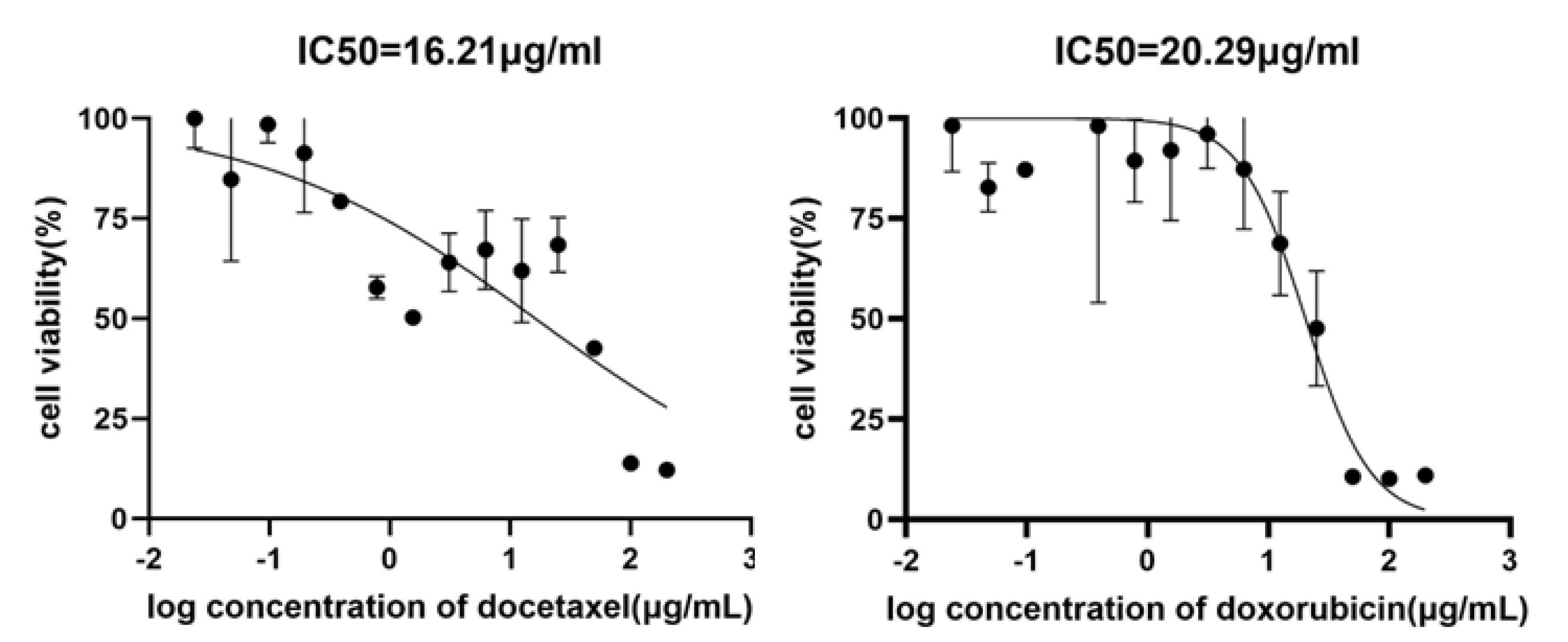
Figure 1.
Effective Concentrations of Docetaxel and Doxorubicin in the A-375 Cell Line after 24 Hours of Incubation Evaluated bythe MTT Test. Results show a dose-dependent decrease in cell viability.
.
Effective Concentrations of Docetaxel and Doxorubicin in the A-375 Cell Line after 24 Hours of Incubation Evaluated bythe MTT Test. Results show a dose-dependent decrease in cell viability.
The Expression of PD-L1, CTLA-4, and VISTA Genes in A-375 Cells Following Chemotherapy Treatment
Following the determination of IC50 values, we examined the expression of the PD-L1, CTLA-4, and VISTA genes when exposed to chemotherapy drugs. For this purpose, the qRT-PCR method was used as previously outlined. One-way ANOVA was used to assess statistical significance (Figure 2).
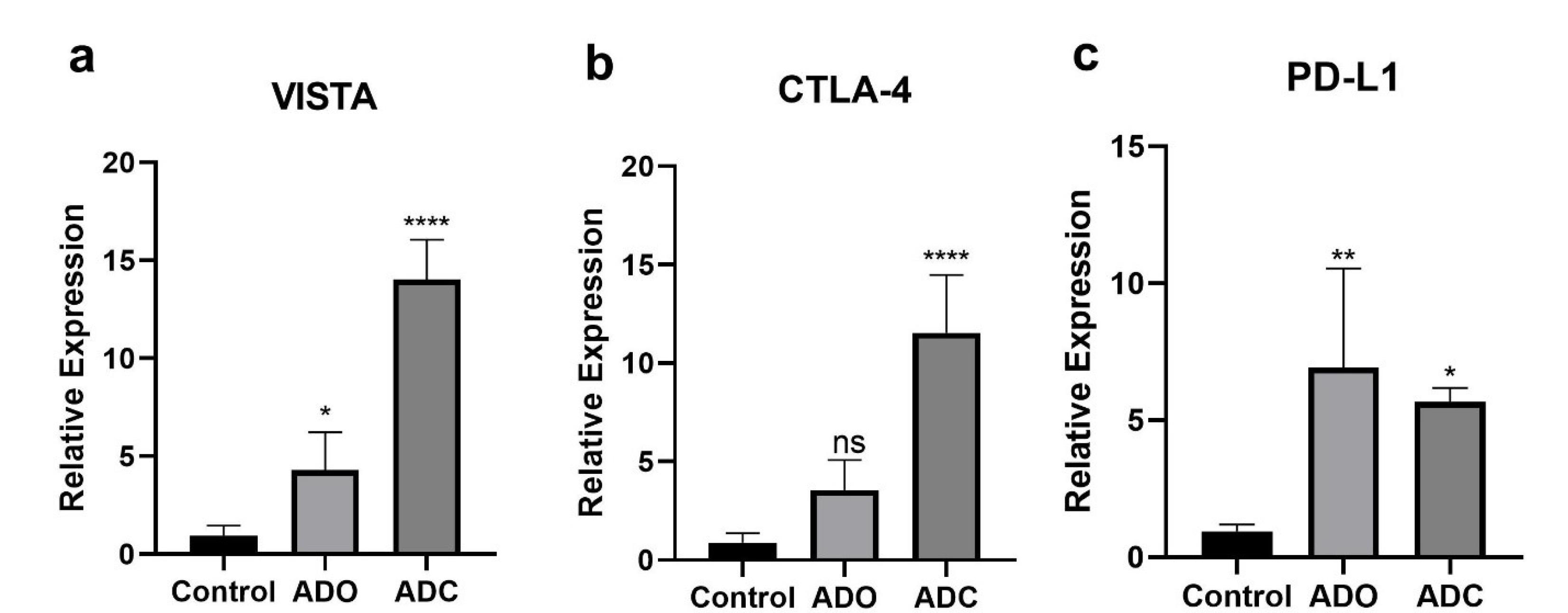
Figure 2.
Relative Expression Levels of Immune Checkpoint Markers (VISTA, CTLA-4, and PD-L1) in A-375 Melanoma Cells Treated with Doxorubicin and Docetaxel Compared to the Control Group. Significant upregulation of VISTA, CTLA-4, and PD-L1 was observed in the treatment groups.
.
Relative Expression Levels of Immune Checkpoint Markers (VISTA, CTLA-4, and PD-L1) in A-375 Melanoma Cells Treated with Doxorubicin and Docetaxel Compared to the Control Group. Significant upregulation of VISTA, CTLA-4, and PD-L1 was observed in the treatment groups.
Discussion
Melanoma has been the subject of extensive research due to its increasing incidence and limited treatment options. When tumor cells recur after chemotherapy, they may become more aggressive due to their enhanced ability to evade the innate and adaptive immune systems of the body.22,23 This evasion can lead to tumor recurrence and metastasis, which may significantly impact patient outcomes.22,23 To reduce mortality rates, it is essential to understand the cellular mechanisms that enable melanoma cells to develop resistance to chemotherapy.13 Immune checkpoints, such as CTLA-4, PD-L1/PD-1, and VISTA, play a crucial role in suppressing immune responses against cancer.13,14 Targeting these checkpoints has shown promise as a therapeutic strategy, as it can improve clinical outcomes by enhancing tumor-specific immune responses.14,24
In this study, we evaluated the effects of two widely used chemotherapy drugs, doxorubicin and docetaxel, on immune checkpoint expression in melanoma using the A-375 cell line. Our findings reveal significant alterations in the expression levels of CTLA-4, VISTA, and PD-L1 following chemotherapy, highlighting important implications for the treatment of melanoma. One of the notable observations is the consistent increase in the expression levels of CTLA-4, VISTA, and PD-L1 in melanoma cells after treatment with both doxorubicin and docetaxel. This upregulation suggests that these immune checkpoints may contribute to immune resistance in melanoma.
Mechanisms Underlying the Upregulation of Immune Checkpoints
The chemotherapy-induced upregulation of CTLA-4, VISTA, and PD-L1 observed in our study may be influenced by several underlying mechanisms. Chemotherapy has been shown to induce a stress response in the tumor microenvironment, triggering the release of cytokines, such as IFN-γ, that can upregulate PD-L1 expression, thereby allowing tumor cells to evade immune detection.14,25 On the other hand, the upregulation of VISTA expression may be associated with chemotherapy-induced hypoxia as hypoxic conditions have been shown to activate hypoxia-inducible factor 2-alpha (HIF-2α), leading to increased VISTA expression that may contribute to immune suppression.13 The increase in CTLA-4 expression, although modest, might still enhance tumor-induced immunosuppression by interacting with immune cells within the tumor microenvironment.13,24 These findings align with those of the previous studies which suggest that chemotherapy can modulate the immune landscape by inducing checkpoint upregulation, which may, in turn, facilitate immune escape.26,27
Clinical Implications and Existing Human Studies
Our findings are consistent with those of the existing human studies demonstrating the clinical significance of PD-L1, VISTA, and CTLA-4 in melanoma. For instance, immune checkpoint inhibitors such as pembrolizumab28 (PD-1 inhibitor) and ipilimumab (CTLA-4 inhibitor) have shown remarkable efficacy in melanoma by reactivating T-cell responses against tumor cells and have been approved by the US FDA for advanced melanoma.29 These studies have also indicated that treatment with PD-1/PD-L1 or CTLA-4 blockade is associated with immune-related adverse events, underscoring the complexity of immune modulation in cancer therapy. Combination therapies using CTLA-4 and PD-1/PD-L1 inhibitors are being actively investigated to assess their efficacy in enhancing tumor control beyond monotherapy.5,29,30 A recent study on melanoma biopsies found that PD-1 and VISTA expression correlated with poor prognosis, suggesting that these markers may be valuable for predicting patient outcomes and tailoring treatment approaches.31,32
Checkpoint Upregulation and Resistance Mechanisms
The upregulation of immune checkpoints, as observed in this study, suggests that tumor cells may use these pathways to develop resistance to chemotherapy. By increasing the expression of CTLA-4, PD-L1, and VISTA, melanoma cells can inhibit T-cell activation and evade immune surveillance, thereby reducing the effectiveness of chemotherapy. This resistance mechanism points to the potential benefits of combining chemotherapy with ICIs. For example, docetaxel has shown promise when combined with PD-L1 or CTLA-4 inhibitors, resulting in improved tumor control and increased survival rates in advanced melanoma.29,30,32,33 Therefore, combining chemotherapy with immune checkpoint inhibitors may serve as an effective strategy for overcoming immune resistance as it could counteract the upregulation of checkpoints induced by chemotherapy.
Recent studies have emphasized the potential for enhanced clinical efficacy through combination therapies involving immune checkpoint inhibitors. For example, Ariyan et al found that adding CTLA-4 blockade to chemotherapy in a murine model of melanoma led to an increased infiltration of CD4 + and CD8 + T cells, suggesting that checkpoint inhibition can synergize with chemotherapy to boost anti-tumor immunity. A subsequent phase II clinical trial in patients with recurrent melanoma confirmed this finding, demonstrating higher response rates and improved progression-free survival compared to treatment with chemotherapy or CTLA-4 blockade alone.34
Limitations and Future Perspectives
This study has several limitations as well. First, our experiments were conducted solely on the A-375 melanoma cell line, which may not cover the diversity of melanoma biology in patients. Additionally, our research primarily examined the short-term effects of chemotherapy, whereas the long-term impact on immune checkpoint expression and potential clinical outcomes requires further investigation. Future studies are required to validate these findings in patient-derived samples and preclinical models. Examining the specific signaling pathways and regulatory elements involved in checkpoint upregulation could also help identify new therapeutic targets and inform strategies for combining chemotherapy with immunotherapy.26,27,35
Conclusion
In conclusion, our study reveals that doxorubicin and docetaxel can induce the upregulation of immune checkpoint molecules in melanoma cells. These findings underscore the complex interplay between chemotherapy and the immune system in the context of melanoma treatment. Understanding these dynamics may guide the development of more effective combination therapies and personalized treatment strategies for melanoma patients, ultimately improving their outcomes and quality of life. Further research is essential to clarify the processes behind these observations and to translate them into clinically relevant approaches.
Ethics statement
The study received approval from the Medical Ethics Committee of Tabriz University of Medical Sciences (IR.TBZMED.VCR.REC.1402.077). The research was conducted in accordance with the principles of the Helsinki Declaration.
Conflict of interests declaration
The authors declare no conflict of interests.
Acknowledgments
We thank the research staff at the Immunology Research Center, Tabriz University of Medical Sciences, for their invaluable assistance with the experimental procedures.
Data availability statement
This article provides a thorough overview of all pertinent information. For additional details or clarification, please contact the corresponding author.
Author contributions
Conceptualization: Mahya Ahmadpour Youshanlui.
Data curation: Mahya Ahmadpour Youshanlui, Zahra Valedkarimi, Ali Jafarizadeh.
Formal analysis: Alireza Abdshah.
Methodology: Mahya Ahmadpour Youshanlui, Sahand Eslami, Hadi Nasiri.
Supervision: Behzad Baradaran.
Validation: Ali Jafarizadeh.
Writing–original draft: Kimia Motlagh Asghari, Arezou Jafari.
Writing–review & editing: Mahya Ahmadpour Youshanlui, Behzad Baradaran.
Consent for publication
Not applicable.
References
- Arnold M, Singh D, Laversanne M, Vignat J, Vaccarella S, Meheus F. Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol 2022; 158(5):495-503. doi: 10.1001/jamadermatol.2022.0160 [Crossref] [ Google Scholar]
- Mahumud RA, Janda M, Soyer HP, Fernández-Peñas P, Mar VJ, Morton RL. Assessing the value of precision medicine health technologies to detect and manage melanoma. Med J Aust 2022; 217(6):275-8. doi: 10.5694/mja2.51696 [Crossref] [ Google Scholar]
- Thiam A, Zhao Z, Quinn C, Barber B. Years of life lost due to metastatic melanoma in 12 countries. J Med Econ 2016; 19(3):259-64. doi: 10.3111/13696998.2015.1115764 [Crossref] [ Google Scholar]
- Siotos C, Grunvald MW, Damoulakis G, Becerra AZ, O’Donoghue CM, Dorafshar AH. Trends in skin melanoma burden: findings from the Global Burden of Disease Study. Eplasty 2022; 22:e9. [ Google Scholar]
- Schadendorf D, van Akkooi ACJ, Berking C, Griewank KG, Gutzmer R, Hauschild A. Melanoma. Lancet 2018; 392(10151):971-84. doi: 10.1016/s0140-6736(18)31559-9 [Crossref] [ Google Scholar]
- Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 2010; 11(2):155-64. doi: 10.1016/s1470-2045(09)70334-1 [Crossref] [ Google Scholar]
- Read RL, Thompson JF. The role of regional chemotherapy for advanced limb melanoma in the era of potentially effective systemic therapies. Melanoma Res 2021; 31(4):290-7. doi: 10.1097/cmr.0000000000000740 [Crossref] [ Google Scholar]
- Shaffer SM, Dunagin MC, Torborg SR, Torre EA, Emert B, Krepler C. Rare cell variability and drug-induced reprogramming as a mode of cancer drug resistance. Nature 2017; 546(7658):431-5. doi: 10.1038/nature22794 [Crossref] [ Google Scholar]
- Wilson MA, Schuchter LM. Chemotherapy for Melanoma. Cancer Treat Res 2016; 167:209-29. doi: 10.1007/978-3-319-22539-5_8 [Crossref] [ Google Scholar]
- Moreira A, Heinzerling L, Bhardwaj N, Friedlander P. Current melanoma treatments: where do we stand?. Cancers (Basel) 2021; 13(2):221. doi: 10.3390/cancers13020221 [Crossref] [ Google Scholar]
- Weber J. Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother 2009; 58(5):823-30. doi: 10.1007/s00262-008-0653-8 [Crossref] [ Google Scholar]
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8(8):793-800. doi: 10.1038/nm730 [Crossref] [ Google Scholar]
- Li N, Yang S, Ren Y, Tai R, Liu H, Wang Y. Chemotherapy induces immune checkpoint VISTA expression in tumor cells via HIF-2alpha. Biochem Pharmacol 2023; 210:115492. doi: 10.1016/j.bcp.2023.115492 [Crossref] [ Google Scholar]
- Allard B, Pommey S, Smyth MJ, Stagg J. Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs. Clin Cancer Res 2013; 19(20):5626-35. doi: 10.1158/1078-0432.Ccr-13-0545 [Crossref] [ Google Scholar]
- Black M, Barsoum IB, Truesdell P, Cotechini T, Macdonald-Goodfellow SK, Petroff M. Activation of the PD-1/PD-L1 immune checkpoint confers tumor cell chemoresistance associated with increased metastasis. Oncotarget 2016; 7(9):10557-67. doi: 10.18632/oncotarget.7235 [Crossref] [ Google Scholar]
- Kaunitz GJ, Cottrell TR, Lilo M, Muthappan V, Esandrio J, Berry S. Melanoma subtypes demonstrate distinct PD-L1 expression profiles. Lab Invest 2017; 97(9):1063-71. doi: 10.1038/labinvest.2017.64 [Crossref] [ Google Scholar]
- Pistillo MP, Carosio R, Grillo F, Fontana V, Mastracci L, Morabito A. Phenotypic characterization of tumor CTLA-4 expression in melanoma tissues and its possible role in clinical response to Ipilimumab. Clin Immunol 2020; 215:108428. doi: 10.1016/j.clim.2020.108428 [Crossref] [ Google Scholar]
-
Rosenbaum SR, Knecht M, Mollaee M, Zhong Z, Erkes DA, McCue PA, et al. FOXD3 regulates VISTA expression in melanoma. Cell Rep 2020;30(2):510-24.e6. doi: 10.1016/j.celrep.2019.12.036.
- Majidi M, Safaee S, Amini M, Baghbanzadeh A, Hajiasgharzadeh K, Hashemzadeh S. The effects of chemotherapeutic drugs on PD-L1 gene expression in breast cancer cell lines. Med Oncol 2021; 38(12):147. doi: 10.1007/s12032-021-01556-0 [Crossref] [ Google Scholar]
- Yang S, Shim MK, Kim WJ, Choi J, Nam GH, Kim J. Cancer-activated doxorubicin prodrug nanoparticles induce preferential immune response with minimal doxorubicin-related toxicity. Biomaterials 2021; 272:120791. doi: 10.1016/j.biomaterials.2021.120791 [Crossref] [ Google Scholar]
- General Cell Collection. Iran. Available from: https://fa.pasteur.ac.ir/uploads/1/2020/May/10/%D8%B1%D8%AF%D9%87%20%D9%87%D8%A7%DB%8C%20%D8%B3%D9%84%D9%88%D9%84%DB%8C.pdf.
- Robert C. Is earlier better for melanoma checkpoint blockade?. Nat Med 2018; 24(11):1645-8. doi: 10.1038/s41591-018-0250-0 [Crossref] [ Google Scholar]
- Karakousis G. Adjuvant therapy for melanoma: how to choose?. Lancet Oncol 2020; 21(3):319-20. doi: 10.1016/s1470-2045(20)30002-4 [Crossref] [ Google Scholar]
- Logtenberg ME, Scheeren FA, Schumacher TN. The CD47-SIRPα immune checkpoint. Immunity 2020; 52(5):742-52. doi: 10.1016/j.immuni.2020.04.011 [Crossref] [ Google Scholar]
- Mandai M, Hamanishi J, Abiko K, Matsumura N, Baba T, Konishi I. Dual faces of IFNγ in cancer progression: a role of PD-L1 induction in the determination of pro- and antitumor immunity. Clin Cancer Res 2016; 22(10):2329-34. doi: 10.1158/1078-0432.Ccr-16-0224 [Crossref] [ Google Scholar]
- Liu W, Zhang L, Xiu Z, Guo J, Wang L, Zhou Y. Combination of immune checkpoint inhibitors with chemotherapy in lung cancer. Onco Targets Ther 2020; 13:7229-41. doi: 10.2147/ott.s255491 [Crossref] [ Google Scholar]
- Hassanian H, Asadzadeh Z, Baghbanzadeh A, Derakhshani A, Dufour A, Rostami Khosroshahi N. The expression pattern of Immune checkpoints after chemo/radiotherapy in the tumor microenvironment. Front Immunol 2022; 13:938063. doi: 10.3389/fimmu.2022.938063 [Crossref] [ Google Scholar]
- Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015; 33(17):1974-82. doi: 10.1200/jco.2014.59.4358 [Crossref] [ Google Scholar]
- Rausch MP, Hastings KT. Immune Checkpoint Inhibitors in the Treatment of Melanoma: From Basic Science to Clinical Application. Exon Publications; 2017. p. 121-42.
- Huang X, Zhang X, Li E, Zhang G, Wang X, Tang T. VISTA: an immune regulatory protein checking tumor and immune cells in cancer immunotherapy. J Hematol Oncol 2020; 13(1):83. doi: 10.1186/s13045-020-00917-y [Crossref] [ Google Scholar]
- Edwards J, Tasker A, Pires da Silva I, Quek C, Batten M, Ferguson A. Prevalence and cellular distribution of novel immune checkpoint targets across longitudinal specimens in treatment-naïve melanoma patients: implications for clinical trials. Clin Cancer Res 2019; 25(11):3247-58. doi: 10.1158/1078-0432.Ccr-18-4011 [Crossref] [ Google Scholar]
- Carlino MS, Larkin J, Long GV. Immune checkpoint inhibitors in melanoma. Lancet 2021; 398(10304):1002-14. doi: 10.1016/s0140-6736(21)01206-x [Crossref] [ Google Scholar]
- Yum JI, Hong YK. Terminating cancer by blocking VISTA as a novel immunotherapy: Hasta la vista, baby. Front Oncol 2021; 11:658488. doi: 10.3389/fonc.2021.658488 [Crossref] [ Google Scholar]
- Ariyan CE, Brady MS, Siegelbaum RH, Hu J, Bello DM, Rand J. Robust antitumor responses result from local chemotherapy and CTLA-4 blockade. Cancer Immunol Res 2018; 6(2):189-200. doi: 10.1158/2326-6066.Cir-17-0356 [Crossref] [ Google Scholar]
- Lao Y, Shen D, Zhang W, He R, Jiang M. Immune checkpoint inhibitors in cancer therapy-how to overcome drug resistance?. Cancers (Basel) 2022; 14(15):3575. doi: 10.3390/cancers14153575 [Crossref] [ Google Scholar]