Int J Drug Res Clin. 2:e20.
doi: 10.34172/ijdrc.2024.e20
Original Article
Effects of Endometrial Features and Uterine Artery Doppler Index Changes in Response to Progesterone Administration on Pregnancy Outcomes in Frozen-Thawed Embryo Transfer
Parvin Hakimi 1  , Nazila Rahnamay Tarighi 2, Ali Abzirakan Aslanduz 2, Shahab Abdi 2, Mahdi Hemmati Ghavshough 3, Elham Eghbali 2, *
, Nazila Rahnamay Tarighi 2, Ali Abzirakan Aslanduz 2, Shahab Abdi 2, Mahdi Hemmati Ghavshough 3, Elham Eghbali 2, * 
Author information:
1Women’s Reproductive Health Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2Medical Radiation Sciences Research Group, Tabriz University of Medical Sciences, Tabriz, Iran
3Medical Faculty, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Background:
Identifying effective factors in the success rate of assisted reproductive technologies can be a promising step. This study examined the effects of changes in endometrial thickness and vascularity following administration of progesterone on pregnancy rates in women who underwent frozen-thawed euploid blastocyst transfer cycles.
Methods:
Overall, 200 infertile women underwent in vitro fertilization (IVF) to treat infertility. All patients received estrogen, and 10 days after the start of estrogen, if the thickness of the endometrium was more than 7 mm, progesterone was started for the patient. A transvaginal ultrasound was performed on the day of starting progesterone, and the thickness of the endometrium and endometrial view, presence of blood flow in zone III of the endometrium, and uterine artery pulsed Doppler indices were evaluated. The second time of ultrasound was performed 3 days after the initiation of progesterone, and the same parameters were checked on the day of transfer of the cleavage embryo. Eight weeks after the transfer of the embryo, a beta-human chorionic gonadotropin test and ultrasound were conducted to check the pregnancy and the presence of a fetal heartbeat.
Results:
Endometrial thickness significantly decreased after progesterone administration, but it was considerably thicker in the pregnant group than in the non-pregnant. After receiving progesterone therapy, noticeable differences were observed in the appearance of the endometrium and the distribution of vascularity in zone III across the groups.
Conclusion:
Endometrial thickness, view, and zone III vascularity were associated with IVF success rate, but there were no significant changes regarding Doppler indices. Whatever change in Doppler indices that occurs after progesterone administration has no significant effect on embryo transfer success rate.
Keywords: Frozen embryo transfer, Endometrial thickness, Endometrial view, Endometrial vascularity, Uterine artery doppler indices
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
The authors received no financial support for this research.
Please cite this article as follows: Hakimi P, Rahnamay Tarighi N, Abzirakan Aslanduz A, Abdi S, Hemmati Ghavshough M, Eghbali E. Effects of endometrial features and uterine artery doppler index changes in response to progesterone administration on pregnancy outcomes in frozen-thawed embryo transfer. Int J Drug Res Clin. 2024; 2: e20. doi: 10.34172/ijdrc.2024.e20
Introduction
Infertility is the absence of unprotected fertility after one year of intercourse and is observed in approximately 14% of couples. Today, there are many methods used to treat infertility patients depending on the cause of infertility. However, the success rate of this process varies according to different factors and conditions. Determining the effectiveness of this fertility process in its success will be a suitable step in treating these patients as well as possible.1
The endometrium is a unique, active, and hormone-responsive tissue that experiences recurring alterations throughout the menstrual cycle, such as growth, differentiation, damage, and regrowth. These alterations are controlled by hormones such as estrogen and progesterone and are aimed at enabling implantation to occur within the brief “implantation window” phase of the menstrual cycle. Therefore, endometrium receptivity is mandatory for successful implantation, even in natural pregnancy or fertility trials.2,3 A limitation of using assisted reproductive technology (ART) to treat infertility is the low implantation rate despite good embryo quality. In other words, the success rate of ectopic fertilization depends not only on the quality of the ovum and sperm but also on the acceptance of the endometrium.4
Many studies have investigated the effect of endometrial thickness on pregnancy success rate in infertile women who are treated with ART. At the same time, the results are also contradictory. Although some studies point out that the hope of pregnancy with ART is affected by endometrial thickness, other studies have not found a relationship between endometrial thickness and the success rate of these methods.5,6
In addition to endometrial thickness, endometrial blood flow is another sonographic parameter that is being evaluated more. To increase the beneficial endometrial interactions of the fetus, the endometrium should become thicker and more vascularized. Blood flow of the endometrium reflects uterine receptivity because the endometrium is the place of embryo implantation. Studies have been conducted to evaluate the receptivity of the endometrium in endometrial and subendometrial blood sources, especially during intrauterine insemination and in vitro fertilization-embryo transfer cycles.7 The results of these studies indicate that the presence of endometrial blood flow on the color Doppler examination may be a sign of improved pregnancy results, but all these studies evaluated the endometrium in the proliferative or estrogen phase rather than examining the endometrium in the luteal phase and after receiving progesterone.8
Most previous studies investigated endometrial thickness on days 10–12 of the proliferative or estrogen phase (the cycle in which new embryos are transferred) and before progesterone injection. Only a few studies have measured the endometrial thickness in the luteal phase or at the time of progesterone initiation of embryo transfer and evaluated its relationship with the pregnancy success rate, the results of which are controversial.6,9,10
It has been shown that uterine artery blood flow velocity has an increasing trend between the early follicular phase and during implantation.11 Endometrial receptivity is controlled by uterine perfusion; therefore, uterine blood disruption may lead to implantation failure in infertile patients undergoing in vitro fertilization (IVF) procedures.12,13 Previous studies have shown that an increased pulsatility index (PI) of the uterine artery is associated with a lower fertilization rate during IVF cycles.14 However, most studies in patients with implantation failure have investigated uterine perfusion during the follicular phase, while little information is available on the luteal phase.
As mentioned, there are limited and conflicting studies on the grayscale and Doppler endometrial changes after progesterone administration. In addition, in our infertility department, routine ultrasound was performed on the day of starting progesterone and the day of embryo transfer for patients undergoing IVF. Accordingly, it was decided to evaluate the effect of progesterone administration on endometrial thickness and vascularity and its relationship with pregnancy success in frozen embryo transfer (FET) cycles.
Methods
Study Population
This study was conducted on 200 patients between February 2021 and February 2023 at Al-Zahra hospital of Tabriz University of Medical Sciences, which was approved by the Research Ethics Committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1401.139). All participants signed a written consent form for participation in the project. The study was performed in accordance with national guidelines and regulations and the Declaration of Helsinki.
Eligibility Criteria
The inclusion criteria were infertile women aged between 20 and 40 years, women undergoing IVF for the first or second time, women with a body mass index less than 30 kg/m2, and those who provided informed consent to participate in the study. However, women above the age of 40 years or those who had a history of endometriosis, endometrial polyps, submucosal uterine leiomyoma, or adenomyosis, as well as women who used donated ovum or embryo, were excluded from the study. Individuals whose endometrial thickness was less than 7 mm on days 9–10 were excluded as well.
Study Implementation Method
All participants were prescribed a standardized hormone protocol for endometrial preparation. This involved starting with 2 mg of estradiol tablets per day from the first day of the menstrual cycle, followed by an increase to two tablets of estradiol 2 mg for three days and then three tablets of estradiol 2 mg daily until days 9–10 when the patient underwent transvaginal ultrasound. Progesterone treatment was initiated if the endometrial thickness was ≥ 7 mm. If the thickness was < 7 mm, the patient continued to receive estrogen (in the form of pills or vaginal cream) and underwent serial vaginal ultrasound examinations until the thickness reached at least 7 mm.
To administer progesterone, 75 mg of progesterone was administered intramuscularly on the first day, followed by an intramuscular injection of 100 mg of progesterone for two days. On the day of embryo transfer, the patient was referred for transvaginal ultrasound. After ultrasound and data recording, two to three cleavage-stage embryos (i.e., embryos frozen on the third day of development) were transferred to the uterus after warming. A 4-9 MHz vaginal probe of the GE E6 ultrasound machine was used for transvaginal ultrasound by an expert radiologist. A midsagittal section of the uterus was obtained to visualize the cervix in the field. The greatest distance in the endometrial-myometrial junction at the anterior and posterior walls of the uterus was recorded as the endometrial thickness.
An Applebaum uterine scoring system was utilized to assess endometrial vascularity. The endometrial and periendometrial regions were categorized into four zones (zone I: a 2-mm thick area surrounding the hyperechoic outer layer of the endometrium, zone II: the outer endometrial hyperechoic layer, zone III: the inner endometrial hypoechoic layer, and zone IV: the cavity of the endometrium).15 Based on the results of the ultrasound examination, the distribution of vascularity in zone III of the endometrium, which is known as the functional layer of the endometrium, was determined for each patient and recorded on the checklist.
With women lying in a supine position, Doppler ultrasound was performed using the same 4-9 MHz vaginal probe of the GE E6 ultrasound machine. After finding the external iliac artery and the uterine artery medial to it, waveforms of flow velocity were obtained from each uterine artery near the external iliac artery before branching of the uterine artery, as described previously.16 Right uterine artery resistance index, left uterine artery resistance index (LURI), right uterine artery PI (RUPI), and left uterine artery PI (LUPI) were measured using the automated method.
To determine the success of pregnancy after embryo transfer, patients were divided according to whether they had a positive beta human chorionic gonadotropin (βhCG) test or fetal heart rate in the ultrasound of the fetus (Figure 1).
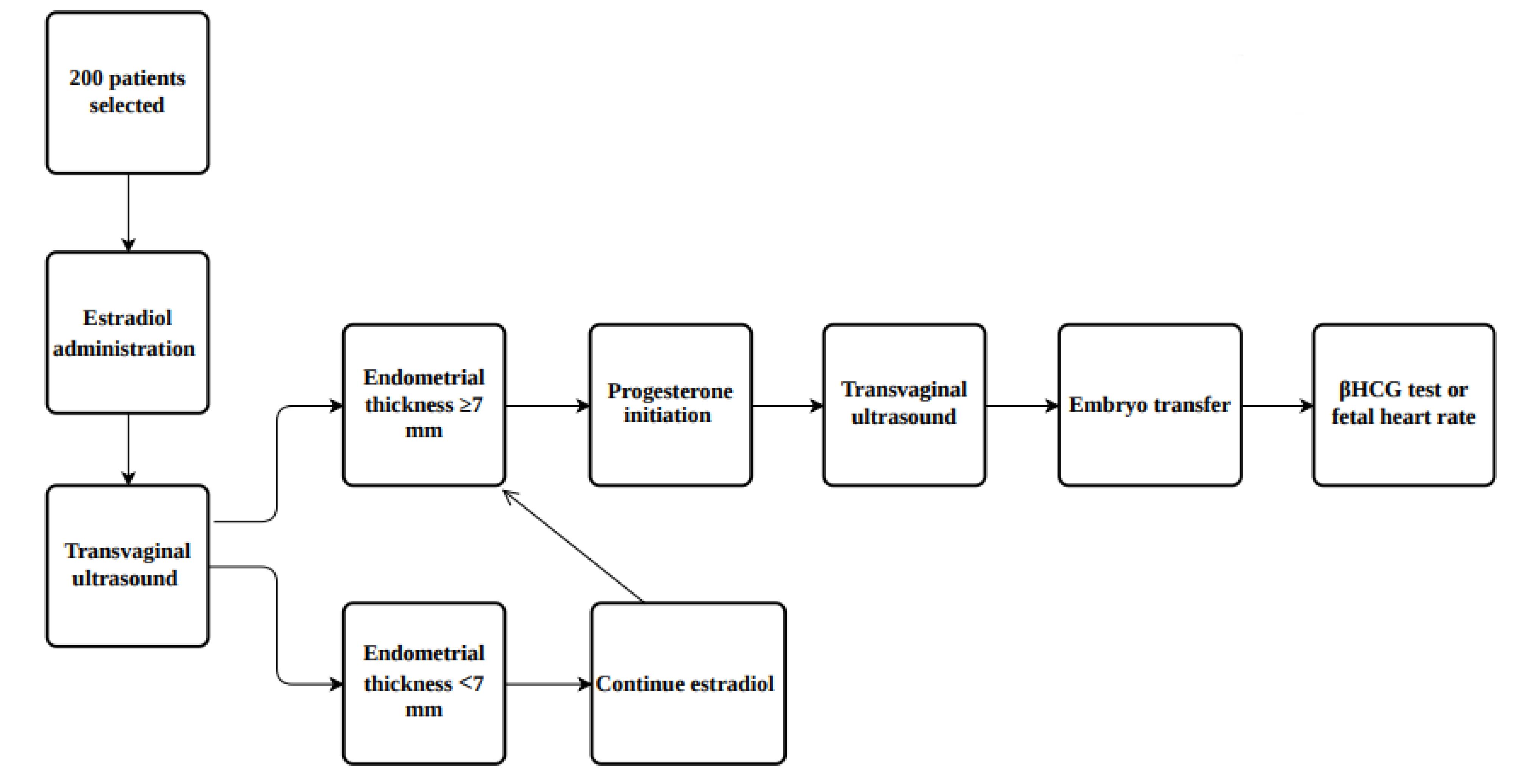
Figure 1.
Diagram Summarizing the Study Design
.
Diagram Summarizing the Study Design
Statistical Analysis
We used the Statistical Package for Social Sciences Statistics, version 23 (IBM, NY, USA) for data analysis. Continuous and categorical variables were reported as means ± standard deviations, as well as numbers and percentages, respectively. Independent samples t tests and chi-square tests were used to compare quantitative and qualitative variables, respectively. In addition, Fisher’s exact test was utilized, if applicable. A P value less than 0.05 was considered to indicate statistical significance.
Results
Patients’ Demographic Data
In general, 200 infertile women undergoing FET were included in this study (81 and 119 people with positive- and negative-pregnancy results, respectively). Pregnancy-positive patients were significantly younger than pregnancy-negative patients (P = 0.005). There were no significant differences in the duration of infertility (P = 0.54) or the number of IVF attempts (P = 0.44) between the groups (P = 0.005, Table 1).
Table 1.
Baseline Characteristics of Total Participants Based on Pregnancy Outcome
|
Variable
|
Total
|
Pregnant
|
Non-pregnant
|
P
Value
|
| Age (y) |
34.75 ± 5.93 |
33.40 ± 4.90 |
35.67 ± 6.40 |
0.005 |
| Duration of infertility (y) |
6.71 ± 4.29 |
4.22 ± 0.47 |
6.86 ± 4.36 |
0.536 |
| Number of previous IVF attempts |
Once |
63 |
28 |
35 |
0.441 |
| Twice |
137 |
53 |
84 |
Note. IVF: In vitro fertilization. The values are presented as means ± standard deviations.
Endometrial Thickness
The overall endometrial thickness significantly decreased after progesterone administration (before progesterone administration: 9.66 ± 1.69 mm vs. 8.86 ± 1.76 mm after progesterone administration, P < 0.001). In addition, there were significant changes in endometrial thickness before progesterone administration between the pregnant and nonpregnant groups (10.11 ± 1.97 mm and 9.36 ± 1.39 mm, respectively, P = 0.004). Endometrial thickness was still significantly greater in the pregnant group (9.26 ± 1.94 mm) than in the nonpregnant group (8.59 ± 1.58 mm) after progesterone administration (P = 0.011, Figure 2).
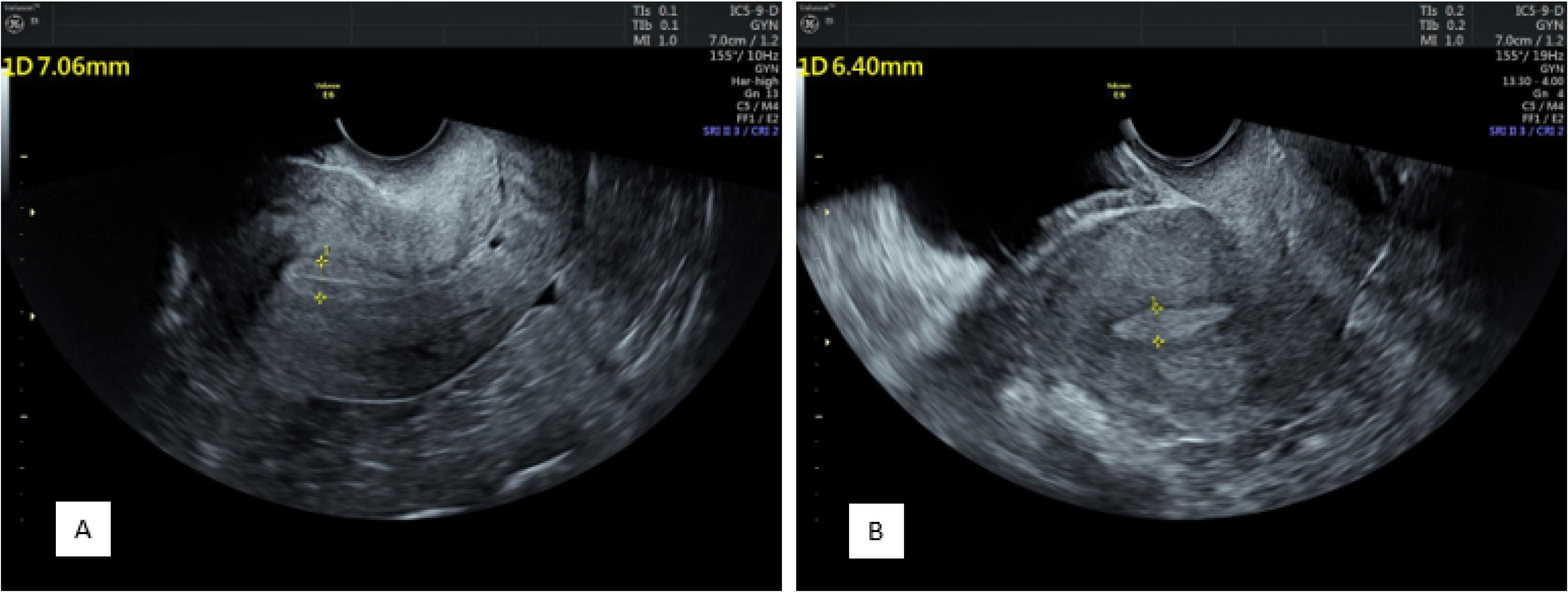
Figure 2.
Endometrial Thickness and View Before (A) and After (B) Progesterone Administration Showing a Decrease in Thickness and Changing From a Clear Three-Layer View Containing a Central Hyperechoic Line Surrounded by Two Hypoechoic Layers to an Endometrium Without Distinguishable Layers
.
Endometrial Thickness and View Before (A) and After (B) Progesterone Administration Showing a Decrease in Thickness and Changing From a Clear Three-Layer View Containing a Central Hyperechoic Line Surrounded by Two Hypoechoic Layers to an Endometrium Without Distinguishable Layers
Endometrial Views
Before progesterone administration, 13 patients had an endometrium without layers, 30 had three unclear layers, and 157 had three clear layers. After progesterone administration, the endometrium consisted of 106, 38, and 56 without layers, three unclear layers, and three clear layers, respectively (P < 0.001, Figure 2).
Comparing the appearance of the endometrium before the administration of progesterone in the two groups, in the positive group, 9 were without layers, 14 had three unclear layers, and 58 had three clear layers, and in the negative group, 4 were without layers, 16 had three unclear layers, and 99 had three clear layers (P = 0.056).
Based on the comparison results regarding the appearance of the endometrium after the administration of progesterone in the two groups, in the positive group, 33 were without layers, 9 had three unclear layers, and 39 had three clear layers. In addition, in the negative group, 67 were without layers, 29 had three unclear layers, and 23 had three clear layers (P = 0.002).
Endometrial Zone III Vascularity
According to the comparison results related to zone III vascularity distribution in patients before progesterone administration, multifocal distribution was observed in 166 patients, and sparse distribution was found in 34. After administration, multifocal distribution and sparse distribution were detected in 111 and 89 patients, respectively (P = 0.001).
As regards zone III vascularity distribution before progesterone administration in the positive pregnancy group, multifocal distribution and sparse distribution were found in 76 and 5 patients, respectively. In the negative pregnancy group, multifocal distribution was observed in 90 patients, and sparse distribution was detected in 29 patients (P = 0.001).
Concerning zone III vascularity distribution after progesterone administration in the positive pregnancy group, both types of distribution were found in 56 (multifocal distribution) and 25 (sparse distribution) patients, respectively. In the negative pregnancy group, multifocal distribution and sparse distribution were observed in 55 and 64 patients, respectively (P = 0.001, Figure 3).
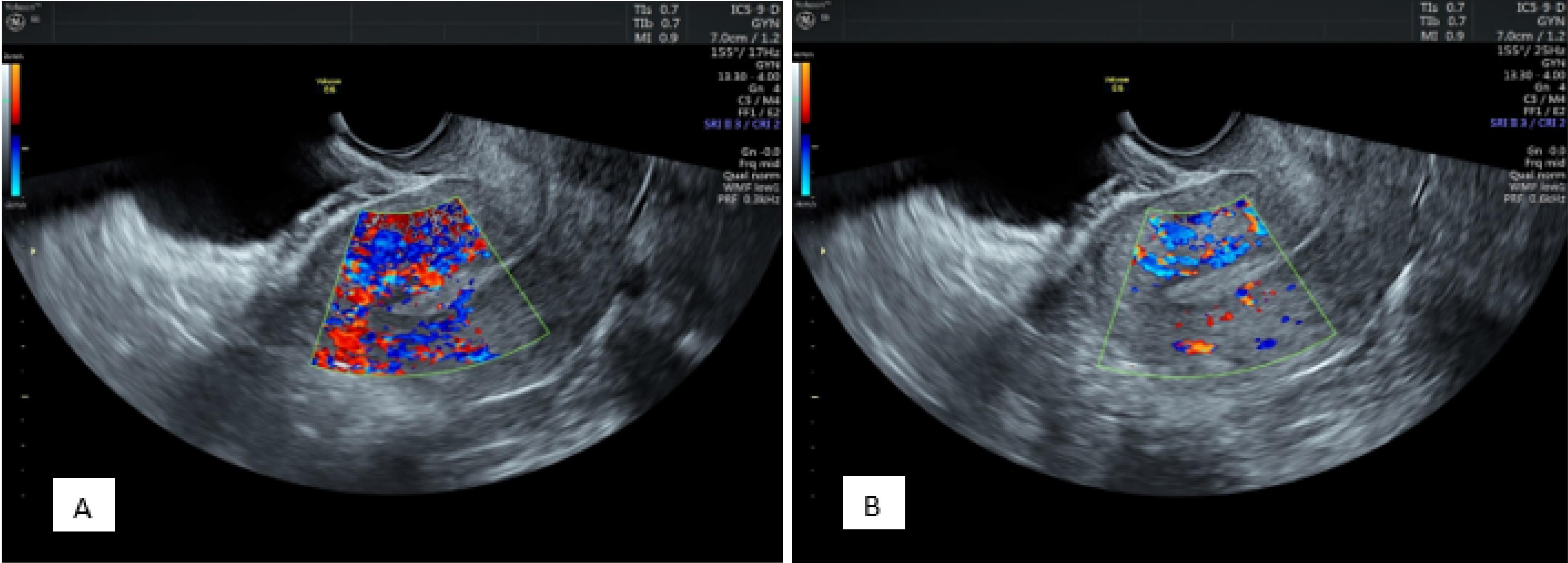
Figure 3.
Endometrial Zone III Vascularity Distribution Before (A) and After (B) Progesterone 9 Administration Demonstrating a Decrease in Multi-focal Distribution and the Sparse Vascularity Being 10 More Prominent
.
Endometrial Zone III Vascularity Distribution Before (A) and After (B) Progesterone 9 Administration Demonstrating a Decrease in Multi-focal Distribution and the Sparse Vascularity Being 10 More Prominent
Uterine Artery Doppler Ultrasound
The calculation software in the ultrasound machine preset was used to measure the PI and resistance index (RI) of the uterine arteries. They were computed using peak systolic velocity (PSV) and end-diastolic velocity (EDV) with the following equations:
PI = PSV-EDV/mean maximum flow velocity, RI = PSV-EDV/PSV
The results regarding this parameter are provided in Table 2. There was no significant difference in right uterine artery RI values after progesterone administration. This value was significantly lower in women who had a successful pregnancy before the administration of progesterone than in women who had an unsuccessful pregnancy, but this difference was not significant after its administration. In the LURI examination, no significant difference was found in the LURI values after progesterone administration. This value was significantly lower in women who had a successful pregnancy before the administration of progesterone than in women who had an unsuccessful pregnancy. After the administration of progesterone, the LURI was significantly lower in women who had a successful pregnancy than women with an unsuccessful pregnancy.
Table 2.
Results of Doppler Ultrasound of the Uterine Arteries Before and After Progesterone Administration Among Total Participants Based on Pregnancy Outcome
|
|
Before
|
After
|
P
Value
|
| RURI |
Total |
0.98 ± 0.85 |
0.88 ± 0.19 |
0.1052 |
| Pregnant |
0.80 ± 0.06 |
0.86 ± 0.09 |
< 0.0001 |
| Non-pregnant |
1.00 ± 0.09 |
0.9 ± 0.23 |
< 0.0001 |
|
P value |
< 0.0001 |
0.138 |
|
| LURI |
Total |
1.00 ± 0.83 |
0.94 ± 0.36 |
0.34 |
| Pregnant |
0.83 ± 0.06 |
0.085 ± 0.07 |
< 0.0001 |
| Non-pregnant |
1.00 ± 0.09 |
1.00 ± 0.46 |
1.00 |
|
P value |
< 0.0001 |
< 0.0001 |
|
| RUPI |
Total |
2.21 ± 0.72 |
2.58 ± 1.19 |
< 0.001 |
| Pregnant |
2.1 ± 0.44 |
2.58 ± 0.92 |
< 0.001 |
| Non-pregnant |
2.29 ± 0.85 |
2.34 ± 1.59 |
0.762 |
|
P value |
0.065 |
0.221 |
|
| LUPI |
Total |
2.35 ± 0.96 |
2.61 ± 1.15 |
< 0.001 |
| Pregnant |
2.59 ± 0.29 |
2.93 ± 0.45 |
< 0.0001 |
| Non-pregnant |
2.38 ± 0.11 |
2.73 ± 1.27 |
0.003 |
|
P value |
< 0.0001 |
0.175 |
|
Note. RURI: Right uterine artery resistive index; LURI: Left uterine artery resistive index; RUPI: Right uterine artery pulsatility index; LUPI: Left uterine artery pulsatility index. The values are expressed as means ± standard deviations.
Another investigated factor in the present study was LUPI and RUPI. There was a significant increase in RUPI values after progesterone administration. This value was not significant in women who had a successful pregnancy before and after the administration of progesterone than in women who had an unsuccessful pregnancy. In the LUPI examination, a significant difference was observed in the LUPI values after progesterone administration. This value was significantly higher in women who had a successful pregnancy before the administration of progesterone than in women who had an unsuccessful pregnancy. After the administration of progesterone, the LUPI in women who had a successful pregnancy was not significantly higher than women with an unsuccessful pregnancy (Figure 4).
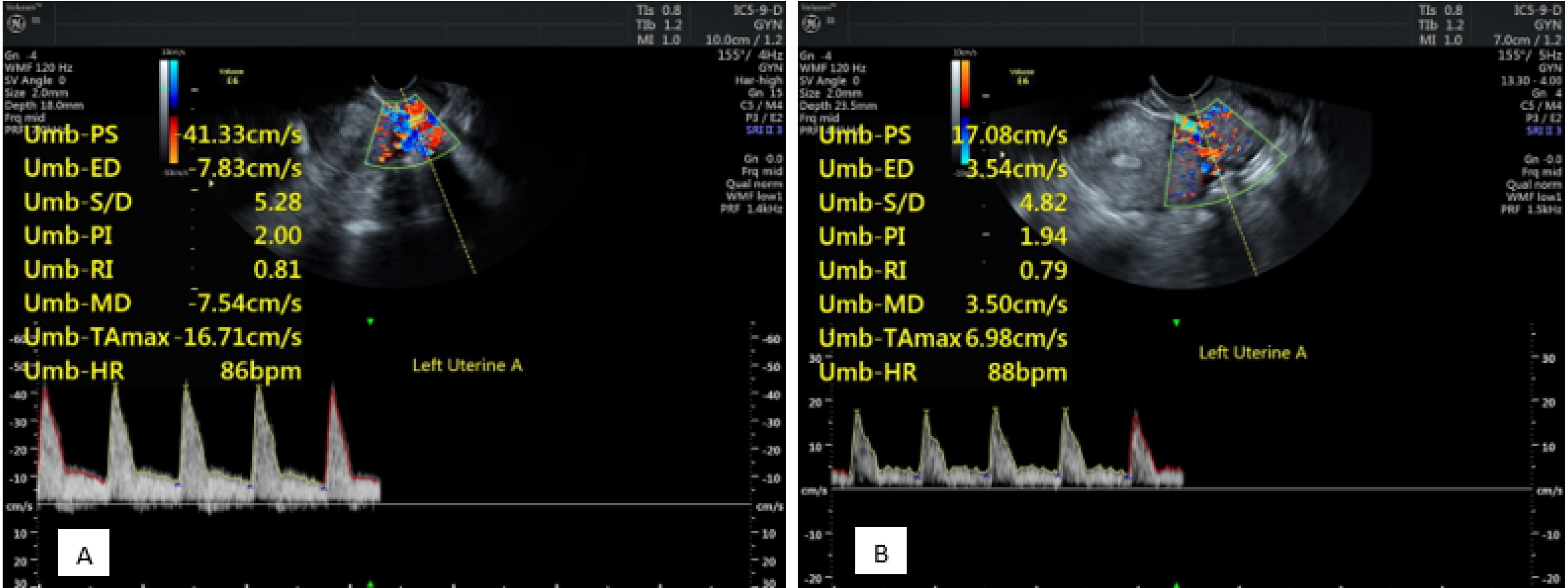
Figure 4.
Left Uterine Artery Pulsed Doppler Investigation Before (A) and After (B) Progesterone Administration Showing a Decrease in Resistance Index and Pulsatility Index
.
Left Uterine Artery Pulsed Doppler Investigation Before (A) and After (B) Progesterone Administration Showing a Decrease in Resistance Index and Pulsatility Index
Discussion
Our findings revealed that among women who underwent IVF, endometrial thickness could be associated with pregnancy outcomes. Additionally, there were significant differences between those who became or did not become pregnant in terms of endometrial vascularity following the administration of progesterone. These findings can help radiologists and gynecologists predict the outcomes of IVF through less invasive procedures. By evaluating the grayscale and Doppler characteristics of the endometrium, clinicians can have a predicting insight into the outcome of fertilization and might modify some of the involved factors by using special pharmaceutical agents to improve the final success rate. These methods prevent invasive procedures such as endometrial biopsy and hysteroscopy. If endometrial progesterone resistance is found, increasing the amount or duration of progesterone administration might be helpful. Reducing estrogen and altering the estrogen-progesterone ratio may also be beneficial. An alternative that may prove useful is the substitution of hormonal preparations in the cycle with natural or modified natural cycles. Further evaluation is needed to determine the effectiveness of these potential changes.
In our study, it was found that younger women had a higher chance of becoming pregnant than middle-aged women. In this context, a systematic review and meta-analysis of 11 335 euploid embryo transfers showed that women under the age of 35 have a higher risk of pregnancy or live birth rate.17 From a genetic perspective, the aneuploidy rate substantially increases in women after the age of 35 years, and the chance of generating a chromosomally normal blastocyst decreases with advancing age.18 This may be attributed to decreased ovarian reserve and reduced oocyte/embryo viability, which are affected by factors such as spindle-assembly checkpoint laxity, telomere shortening, and impaired mitochondrial activity, all of which play an important role in chromosome segregation and embryo competence modulation.18
Based on our findings, the administration of progesterone was associated with a decrease in endometrial thickness, and there were significant differences between pregnant and nonpregnant women. This finding is compatible with the claim of Kovacs et al on 1228 IVF/intracytoplasmic sperm injection cycles that the prediction of pregnancy success with ARTs is influenced by the threshold size of the endometrial thickness.19 Furthermore, Bu et al found that endometrial thickness increased or remained unchanged in most individuals after progesterone was started, and individuals with increased endometrial thickness had better pregnancy outcomes.20 A meta-analysis of 22 studies revealed significantly lower odds (58%) of clinical pregnancy in women with an endometrial thickness ≤ 7 mm than in those with an endometrial thickness > 7 mm.21 The reasons for this finding are under investigation by Casper. When the endometrial thickness is < 7 mm, the functional layer is thin or missing, making embryo implantation adjacent to the spiral vessels and higher vascular distribution and oxygen levels of the basal endometrium. Increased oxygen levels in the basal layer may cause damage compared to low oxygen levels at the endometrial surface.22
Our results also demonstrated that endometrial thickness before and after progesterone in the pregnancy positive group was significantly greater than in the pregnancy negative group. This observation is in line with the findings of a study by Zhao et al, confirming that women with an endometrial thickness > 14 mm had significantly greater implantation and pregnancy success rates than those with an endometrial thickness of 7-14 mm or < 7 mm, indicating that endometrial thickness independently affects pregnancy outcomes.23 In this regard, transvaginal ultrasound revealed that the clear three-layer view was the most common view, which was significantly different between the groups.
There are several possible explanations for these observations. Poor progesterone response may be due to insufficient progesterone effects. One of these reasons may be that some women with infertility have endometrial progesterone receptor deficiency or resistance. There are multiple causes for resistance to the progesterone receptor, including the overexpression of BCL-6 and SIRT-1,24 prolonged endometrial inflammation, polymorphisms of the progesterone receptor gene, changed microRNA expression, and progesterone receptor epigenetic modifications.25,26
Numerous studies, however, have reported that the thickness or volume of the endometrium is not a dependable indicator of successful pregnancy outcomes in ARTs. Accordingly, Yaman et al concluded that endometrial volume and thickness do not predict pregnancy.27 However, it should be noted that they used a three-dimensional ultrasonographic method and included a small sample size of only 65 women.27 Similarly, Barker et al showed that the endometrial thickness before and after taking progesterone did not affect the rate of pregnancy success.28 Kim et al also found that endometrial thickness was not related to pregnancy success or failure among pregnant individuals compared to nonpregnant women.29 Another clinical study on 70 infertile women revealed that endometrial thickness was not a predictor of pregnancy outcomes in FET cycles.30 Notably, most studies were conducted on small samples and not in recent years. Therefore, future large-scale clinical trials and meta-analyses might be necessary to determine the role of endometrial thickness and the relevant cutoff for predicting the rate of pregnancy outcomes. In this context, Haas et al performed a study on 274 FET cycles (2017–2018) and showed that changes in endometrial thickness at the end of the estrogen phase and the day of embryo transfer were inversely related to the rate of pregnancy success, and greater endometrial compaction was associated with higher pregnancy success rates.31 It seems that many studies investigating the relationship between endometrial thickness and pregnancy success have reported conflicting results, which may be due to the different situations of these studies.
Based on these results, it can be assumed that the estrogen-progesterone ratio is important, and excess estrogen effect leads to endometrial growth. This prediction may also explain why implantation and pregnancy rates are lower with fresh hyper-stimulated IVF than with subsequent FET. Elevated estrogen levels beyond physiological norms during certain cycles may disrupt the typical estrogen-progesterone equilibrium within the endometrium, resulting in an inability to achieve proper endometrial thinning. Accordingly, we believe that this is an area for future research.
Before the use of progesterone, there was no significant difference between the two groups of women with successful and unsuccessful pregnancies in terms of endometrial appearance. Conversely, after the administration of progesterone, there was a significant difference between the two groups in terms of endometrial appearance, so that in women with successful pregnancy, there were clear three layers. However, in women whose pregnancy was unsuccessful, the non-layered form was predominant, which conforms to the findings of Yang et al, confirming that the three-line endometrial pattern has a significantly greater clinical pregnancy rate.32 Another study also reported that the triple line pattern, which has a central hyperechoic line surrounded by two hypoechoic layers, is associated with a significantly greater pregnancy rate than an intermediate isoecho pattern, with a reflectivity similar to that of the adjacent myometrium and a vaguely defined central echogenic line, as well as a pattern of having a homogeneous, hyperechogenic endometrium.23 A triple-line pattern of endometrium reflects its proliferation, and such a pattern has been shown to be more associated with pregnancy than the absence of this pattern on the day of hCG injection. The lack of a triple-line pattern could indicate the onset of early endometrial secretory alterations and the passage of the period of maximum endometrial receptivity.33
The conflicting results of these researchers may be in part due to differences in experimental and stimulation methods, heterogeneity between studies, and differences in measurement methods. Most studies examined endometrial thickness and appearance at or after hCG injection and on the day of oocyte retrieval, while other studies evaluated the endometrium on the day of embryo transfer, and even fewer studies assessed endometrial changes on both the days of hCG injection and embryo transfer. Therefore, the favorable timing of endometrial assessment remains undisclosed.
In our study, significant changes were observed in endometrial zone III vascularity before and after progesterone application. Following progesterone administration, there was a significant decrease in multifocality and an increase in sparsity in zone III, and multifocality was significantly higher in those who became pregnant than in those who did not. In this context, Chien et al also reported that the presence of endometrial-subendometrial blood flow, assessed by transvaginal color Doppler, was associated with the implantation rate and pregnancy. In addition, in the presence of blood flow, pregnancy was 5.9 times more common.34 In another study designed to establish a scoring system for endometrial receptivity, the central echo of the endometrium exhibited variations between the groups of individuals who were pregnant and those who were not. However, other factors, such as endometrial blood flow, did not significantly differ between the two groups.35
The findings of the study by Kim et al corroborate our results regarding uterine artery RI and PI. They concluded that the PI and RI were not associated with pregnancy outcomes.29 Similarly, Son et al found that the RI and PI were not significantly associated with pregnancy outcomes in FET cycles.30 However, another study on 169 infertile women demonstrated that although there was no significant difference between RI, PI, and implantation at baseline, there was a large difference between the groups in the duration of ovarian stimulation and on trigger day.36 A comprehensive review and meta-analysis of cohort studies revealed no notable differences in PI or RI when comparing pregnant and non-pregnant populations.37
During the menstrual cycle, there was a decrease in the endometrial artery resistance from the follicular to the mid-luteal phase, and this reduction in resistance continued in the case of fertilization. However, this change did not occur during ART. Drugs, prior pathologies related to the reproductive system of infertile women, and the supraphysiologic estrogen in the blood may disturb the vasculature of the endometrium. Hence, endometrial and sub-endometrial blood flow appears to be lower in ART than in natural cycles.38
The discrepancies found in the literature seem to stem from variations in cycle features, stimulation techniques, transfer cycle types, and the timing of ultrasound during the cycle. More studies are required to explore the impacts of uterine artery Doppler and endometrial perfusion on different aspects influencing fertility. The small number of patients in this study prevented a reasonable comparison of some infertility groups.
Study Limitations
Although large-scale studies have been conducted,39,40 this is one of the studies on a small population of Northwest Iran. Nonetheless, this study had some limitations. First, this was a cross-sectional study with survey data, so there was a possibility of selection bias. Considering that we did not use a random sampling method, there was likely potential sampling bias. Second, it was conducted on a relatively small sample from a specific population in Iran, so the findings cannot be generalized to other races/ethnicities and populations. Third, some confounding factors, such as race/ethnicity and paternal age, were not included since this was a retrospective study. Fourth, we only evaluated the outcomes as to whether pregnancy occurred, while other neonatal and pregnancy outcomes were not investigated in the present study. These limitations may affect the fertility success rate, and the results might not be generalized to other populations. Future studies can use these limitations and address them by expanding the study population to other ethnicities and a greater number of cases and making more inclusive changes to their methods.
Conclusion
Endometrial thickness and vascularity were significantly associated with pregnancy outcomes in those who underwent IVF. Additionally, Doppler and transvaginal ultrasound indices can be helpful markers for predicting the outcomes of IVF. However, the study had a relatively small sample size, and it is recommended that further large-scale observational studies be conducted using previously defined and novel or revised radiological indices for the prediction of IVF outcomes.
Ethics statement
This study was approved by the Research Ethics Committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1401.139). All patients provided written consent for participation in the study.
Conflict of interests declaration
The authors declare that they have no competing interests.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Author contributions
Conceptualization: Elham Eghbali, Nazila Rahnamay Tarighi.
Data curation: Nazila Rahnamay Tarighi, Ali Abzirakan Aslanduz.
Formal analysis: Mahdi Hemmati Ghavshough.
Investigation: Shahab Abdi.
Methodology: Parvin Hakimi.
Project administration: Elham Eghbali, Parvin Hakimi.
Resources: Nazila Rahnamay Tarighi.
Software: Mahdi Hemmati Ghavshough.
Supervision: Elham Eghbali, Parvin Hakimi.
Validation: Ali Abzirakan Aslanduz.
Visualization: Shahab Abdi.
Writing–original draft: Mahdi Hemmati Ghavshough.
Writing–review & editing: Mahdi Hemmati Ghavshough, Ali Abzirakan Aslanduz.
Consent for publication
Not applicable.
References
- Bassil S. Changes in endometrial thickness, width, length and pattern in predicting pregnancy outcome during ovarian stimulation in in vitro fertilization. Ultrasound Obstet Gynecol 2001; 18(3):258-63. doi: 10.1046/j.1469-0705.2001.00502.x [Crossref] [ Google Scholar]
- Moon KS, Richter KS, Levy MJ, Widra EA. Does dilation and curettage versus expectant management for spontaneous abortion in patients undergoing in vitro fertilization affect subsequent endometrial development?. Fertil Steril 2009; 92(5):1776-9. doi: 10.1016/j.fertnstert.2009.05.045 [Crossref] [ Google Scholar]
- Osemwenkha AP, Osaikhuwuomwan JA. Correlation between endometrial thickness and IVF outcome in an African population. Gynecol Obstet 2012; 2(2):119. doi: 10.4172/2161-0932.1000119 [Crossref] [ Google Scholar]
- Yaman C, Ebner T, Sommergruber M, Pölz W, Tews G. Role of three-dimensional ultrasonographic measurement of endometrium volume as a predictor of pregnancy outcome in an IVF-ET program: a preliminary study. Fertil Steril 2000; 74(4):797-801. doi: 10.1016/s0015-0282(00)01493-x [Crossref] [ Google Scholar]
- Schild RL, Knobloch C, Dorn C, Fimmers R, van der Ven H, Hansmann M. Endometrial receptivity in an in vitro fertilization program as assessed by spiral artery blood flow, endometrial thickness, endometrial volume, and uterine artery blood flow. Fertil Steril 2001; 75(2):361-6. doi: 10.1016/s0015-0282(00)01695-2 [Crossref] [ Google Scholar]
- Bu Z, Yang X, Song L, Kang B, Sun Y. The impact of endometrial thickness change after progesterone administration on pregnancy outcome in patients transferred with single frozen-thawed blastocyst. Reprod Biol Endocrinol 2019; 17(1):99. doi: 10.1186/s12958-019-0545-0 [Crossref] [ Google Scholar]
- Kim H. Detection of endometrial subendometrial vasculature on the day of embryo transfer and the prediction of pregnancy during fresh in vitro fertilization cycles. Fertil Steril 2013; 100(3):S514. doi: 10.1016/j.fertnstert.2013.07.231 [Crossref] [ Google Scholar]
- Mercé LT, Barco MJ, Bau S, Troyano J. Are endometrial parameters by three-dimensional ultrasound and power Doppler angiography related to in vitro fertilization/embryo transfer outcome?. Fertil Steril 2008; 89(1):111-7. doi: 10.1016/j.fertnstert.2007.02.029 [Crossref] [ Google Scholar]
- Barker MA, Boehnlein LM, Kovacs P, Lindheim SR. Follicular and luteal phase endometrial thickness and echogenic pattern and pregnancy outcome in oocyte donation cycles. J Assist Reprod Genet 2009; 26(5):243-9. doi: 10.1007/s10815-009-9312-z [Crossref] [ Google Scholar]
- Zilberberg E, Nayot D, Smith RG, Meriano J, Barzilay E, Haas J. Endometrial compaction (decreased thickness) in response to progesterone results in higher ongoing pregnancy rate. Fertil Steril 2019; 112(3):e89-90. doi: 10.1016/j.fertnstert.2019.07.355 [Crossref] [ Google Scholar]
- Lloyd-Davies C, Collins SL, Burton GJ. Understanding the uterine artery Doppler waveform and its relationship to spiral artery remodelling. Placenta 2021; 105:78-84. doi: 10.1016/j.placenta.2021.01.004 [Crossref] [ Google Scholar]
- Banker M, Dyer S, Chambers GM, Ishihara O, Kupka M, de Mouzon J. International Committee for Monitoring Assisted Reproductive Technologies (ICMART): world report on assisted reproductive technologies, 2013. Fertil Steril 2021; 116(3):741-56. doi: 10.1016/j.fertnstert.2021.03.039 [Crossref] [ Google Scholar]
- Ke X, Liang XF, Lin YH, Wang F. Pregnancy prediction via ultrasound-detected endometrial blood for hormone replacement therapy-frozen embryo transfer: a prospective observational study. Reprod Biol Endocrinol 2023; 21(1):112. doi: 10.1186/s12958-023-01164-9 [Crossref] [ Google Scholar]
- Salmeri N, Farina A, Candiani M, Dolci C, Bonavina G, Poziello C. Endometriosis and impaired placentation: a prospective cohort study comparing uterine arteries Doppler pulsatility index in pregnancies of patients with and without moderate-severe disease. Diagnostics (Basel) 2022; 12(5):1024. doi: 10.3390/diagnostics12051024 [Crossref] [ Google Scholar]
- Khan MS, Shaikh A, Ratnani R. Ultrasonography and Doppler study to predict uterine receptivity in infertile patients undergoing embryo transfer. J Obstet Gynaecol India 2016; 66(Suppl 1):377-82. doi: 10.1007/s13224-015-0742-5 [Crossref] [ Google Scholar]
- Campbell S, Diaz-Recasens J, Griffin DR, Cohen-Overbeek TE, Pearce JM, Willson K. New doppler technique for assessing uteroplacental blood flow. Lancet 1983; 1(8326 Pt 1):675-7. doi: 10.1016/s0140-6736(83)91970-0 [Crossref] [ Google Scholar]
- Vitagliano A, Paffoni A, Viganò P. Does maternal age affect assisted reproduction technology success rates after euploid embryo transfer? A systematic review and meta-analysis. Fertil Steril 2023; 120(2):251-65. doi: 10.1016/j.fertnstert.2023.02.036 [Crossref] [ Google Scholar]
- Ubaldi FM, Cimadomo D, Vaiarelli A, Fabozzi G, Venturella R, Maggiulli R. Advanced maternal age in IVF: still a challenge? The present and the future of its treatment. Front Endocrinol (Lausanne) 2019; 10:94. doi: 10.3389/fendo.2019.00094 [Crossref] [ Google Scholar]
- Kovacs P, Matyas S, Boda K, Kaali SG. The effect of endometrial thickness on IVF/ICSI outcome. Hum Reprod 2003; 18(11):2337-41. doi: 10.1093/humrep/deg461 [Crossref] [ Google Scholar]
- Bu Z, Yang X, Song L, Kang B, Sun Y. The impact of endometrial thickness change after progesterone administration on pregnancy outcome in patients transferred with single frozen-thawed blastocyst. Reprod Biol Endocrinol 2019; 17(1):99. doi: 10.1186/s12958-019-0545-0 [Crossref] [ Google Scholar]
- Kasius A, Smit JG, Torrance HL, Eijkemans MJ, Mol BW, Opmeer BC. Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-analysis. Hum Reprod Update 2014; 20(4):530-41. doi: 10.1093/humupd/dmu011 [Crossref] [ Google Scholar]
- Casper RF. It’s time to pay attention to the endometrium. Fertil Steril 2011; 96(3):519-21. doi: 10.1016/j.fertnstert.2011.07.1096 [Crossref] [ Google Scholar]
- Zhao J, Zhang Q, Li Y. The effect of endometrial thickness and pattern measured by ultrasonography on pregnancy outcomes during IVF-ET cycles. Reprod Biol Endocrinol 2012; 10:100. doi: 10.1186/1477-7827-10-100 [Crossref] [ Google Scholar]
- Yoo JY, Kim TH, Fazleabas AT, Palomino WA, Ahn SH, Tayade C. KRAS activation and over-expression of SIRT1/BCL6 contributes to the pathogenesis of endometriosis and progesterone resistance. Sci Rep 2017; 7(1):6765. doi: 10.1038/s41598-017-04577-w [Crossref] [ Google Scholar]
- Hu M, Li J, Zhang Y, Li X, Brännström M, Shao LR. Endometrial progesterone receptor isoforms in women with polycystic ovary syndrome. Am J Transl Res 2018; 10(8):2696-705. [ Google Scholar]
- Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet Gynecol Scand 2017; 96(6):623-32. doi: 10.1111/aogs.13156 [Crossref] [ Google Scholar]
- Yaman C, Ebner T, Sommergruber M, Pölz W, Tews G. Role of three-dimensional ultrasonographic measurement of endometrium volume as a predictor of pregnancy outcome in an IVF-ET program: a preliminary study. Fertil Steril 2000; 74(4):797-801. doi: 10.1016/s0015-0282(00)01493-x [Crossref] [ Google Scholar]
-
Li X, Peng Y, Mao Y, Li Y, Gong F, Ouyang Y. Endometrial receptivity change: ultrasound evaluation on ovulation day and transplantation day during the natural frozen embryo transfer cycle. Frontiers in Endocrinology 2023;14. doi: 10.3389/fendo.2023.1118044.
- Kim A, Jung H, Choi WJ, Hong SN, Kim HY. Detection of endometrial and subendometrial vasculature on the day of embryo transfer and prediction of pregnancy during fresh in vitro fertilization cycles. Taiwan J Obstet Gynecol 2014; 53(3):360-5. doi: 10.1016/j.tjog.2013.05.007 [Crossref] [ Google Scholar]
- Son JB, Jeong JE, Joo JK, Na YJ, Kim CW, Lee KS. Measurement of endometrial and uterine vascularity by transvaginal ultrasonography in predicting pregnancy outcome during frozen-thawed embryo transfer cycles. J Obstet Gynaecol Res 2014; 40(6):1661-7. doi: 10.1111/jog.12406 [Crossref] [ Google Scholar]
-
Haas J, Smith R, Zilberberg E, Nayot D, Meriano J, Barzilay E, et al. Endometrial compaction (decreased thickness) in response to progesterone results in optimal pregnancy outcome in frozen-thawed embryo transfers. Fertil Steril 2019;112(3):503-9.e1. doi: 10.1016/j.fertnstert.2019.05.001.
- Yang W, Zhang T, Li Z, Ren X, Huang B, Zhu G. Combined analysis of endometrial thickness and pattern in predicting clinical outcomes of frozen embryo transfer cycles with morphological good-quality blastocyst: a retrospective cohort study. Medicine (Baltimore) 2018; 97(2):e9577. doi: 10.1097/md.0000000000009577 [Crossref] [ Google Scholar]
- Bourgain C, Devroey P. The endometrium in stimulated cycles for IVF. Hum Reprod Update 2003; 9(6):515-22. doi: 10.1093/humupd/dmg045 [Crossref] [ Google Scholar]
- Chien LW, Au HK, Chen PL, Xiao J, Tzeng CR. Assessment of uterine receptivity by the endometrial-subendometrial blood flow distribution pattern in women undergoing in vitro fertilization-embryo transfer. Fertil Steril 2002; 78(2):245-51. doi: 10.1016/s0015-0282(02)03223-5 [Crossref] [ Google Scholar]
- Zhang CH, Chen C, Wang JR, Wang Y, Wen SX, Cao YP. An endometrial receptivity scoring system basing on the endometrial thickness, volume, echo, peristalsis, and blood flow evaluated by ultrasonography. Front Endocrinol (Lausanne) 2022; 13:907874. doi: 10.3389/fendo.2022.907874 [Crossref] [ Google Scholar]
- Silva Martins R, Helio Oliani A, Vaz Oliani D, Martinez de Oliveira J. Subendometrial resistence and pulsatility index assessment of endometrial receptivity in assisted reproductive technology cycles. Reprod Biol Endocrinol 2019; 17(1):62. doi: 10.1186/s12958-019-0507-6 [Crossref] [ Google Scholar]
- Wu J, Sheng J, Wu X, Wu Q. Ultrasound-assessed endometrial receptivity measures for the prediction of in vitro fertilization-embryo transfer clinical pregnancy outcomes: a meta-analysis and systematic review. Exp Ther Med 2023; 26(3):453. doi: 10.3892/etm.2023.12152 [Crossref] [ Google Scholar]
- Zebitay AG, Tutumlu M, Verit FF, Ilhan GK, Gungor ES, Cetin O. A comparative analysis of arterial blood flow in unexplained infertility, tubal infertility and fertile groups. Gynecol Endocrinol 2016; 32(6):442-5. doi: 10.3109/09513590.2015.1126709 [Crossref] [ Google Scholar]
- Mahutte N, Hartman M, Meng L, Lanes A, Luo ZC, Liu KE. Optimal endometrial thickness in fresh and frozen-thaw in vitro fertilization cycles: an analysis of live birth rates from 96,000 autologous embryo transfers. Fertil Steril 2022; 117(4):792-800. doi: 10.1016/j.fertnstert.2021.12.025 [Crossref] [ Google Scholar]
- Xu J, Zhang S, Jin L, Mao Y, Shi J, Huang R. The effects of endometrial thickness on pregnancy outcomes of fresh IVF/ICSI embryo transfer cycles: an analysis of over 40,000 cycles among five reproductive centers in China. Front Endocrinol (Lausanne) 2021; 12:788706. doi: 10.3389/fendo.2021.788706 [Crossref] [ Google Scholar]