Int J Drug Res Clin. 2:e27.
doi: 10.34172/ijdrc.2024.e27
Original Article
The Inhibition of CD73 in Conjunction With the Blockade of Apoptosis Inhibitors Significantly Impedes the Advancement of Cancer via Pro-Apoptotic Mechanisms
Maryam Farajzadeh 1  , Mohammad-Salar Hosseini 2, Mehran Mozaffari 3, Farzaneh Sadat Eshaghi 2, Fatemeh Alian 2, Sakineh Hajebrahimi 4, 5, Sanam Nami 2, Farhad Jadidi-Niaragh 2, 3, 6, *
, Mohammad-Salar Hosseini 2, Mehran Mozaffari 3, Farzaneh Sadat Eshaghi 2, Fatemeh Alian 2, Sakineh Hajebrahimi 4, 5, Sanam Nami 2, Farhad Jadidi-Niaragh 2, 3, 6, * 
Author information:
1Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran
2Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
3Research Center for Integrative Medicine in Aging, Aging Research Institute, Tabriz University of Medical Sciences, Tabriz, Iran
4Urology Department at Helsinki University, Helsinki, Finland
5Iranian Center of Excellence of Joanna Briggs Institute of Adelaide University, Australia
6Department of Immunology, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Background:
Effective cancer treatments are among the most challenging research goals in the field. Cancer cells have diverse characteristics, including the ability to suppress antitumor immune responses and resistance to apoptosis. The upregulation of CD73 in cancer cells has been suggested in recent studies, promoting proliferation, angiogenesis, and metastasis and suppressing immune functions. On the other hand, BV6 can induce apoptosis in cancer cells by suppressing apoptosis inhibitors. Therefore, this study aimed to explore the cancer treatment potential of BV6 and anti-CD73 agents.
Methods:
This study was conducted on cancer cell lines, including CT26 (colon cancer) and 4T1 (breast cancer). Cancer cells were treated with anti-CD73 small interfering ribonucleic acid (siRNA) molecules and BV6 drugs. The effect of treatment on cell viability was evaluated using 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyl tetrazolium bromide assay and apoptosis test. In addition, the effect of treatment on the expression of target apoptosis-related genes was studied with a real-time polymerase chain reaction assay.
Results:
It was revealed that delivery of anti-CD73 siRNA molecules along with BV6 to cancer cells significantly induced cell death. Although the impact of anti-CD73 siRNA monotherapy was non-significant, the combined treatment significantly decreased the expression of genes involved in cell survival while increasing the expression of apoptosis-promoting genes.
Conclusion:
The findings of this study suggest the combined treatment of cancer cells using anti-CD73 siRNA molecules and BV6 as an effective anticancer intervention in vitro. Further studies should be conducted to determine its effectiveness and safety.
Keywords: BV6, CD73, Cancer immunotherapy, Combination therapy, Small interfering RNA
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
This study is financial y purported by the grant number 66443 from the Tabriz University of Medical Sciences.
Please cite this article as follows: Farajzadeh M, Hosseini MS, Mozaffari M, Eshaghi FS, Alian F, Hajebrahimi S, et al. The inhibition of CD73 in conjunction with the blockade of apoptosis inhibitors significantly impedes the advancement of cancer via pro-apoptotic mechanisms. Int J Drug Res Clin. 2024; 2: e27. doi: 10.34172/ijdrc.2024.e27
Introduction
Cancer is a worldwide threat, accounting for 7.6 million lost lives annually, which is predicted to rise to over 13 million by 2030. With industrialization, lifestyle changes, and the consequent increase in environmental pollutants, the cancer rate is rising.1 Chemotherapy, radiotherapy, surgery –or a combination of these treatments– are the most common treatments against cancers. However, these treatment methods have not had acceptable results in many patients and have limitations, such as high costs for the patient or the healthcare system, life-limiting side effects, and inefficiency in treating more advanced cancer types.2,3 The ineffectiveness of these treatment methods has led researchers to look for novel and more effective treatment methods. Hence, numerous approaches in immunotherapy have been developed to combat cancer.
The Smac mimetic BV6 has been employed as a selective inhibitor of apoptosis protein (IAP) targeting agent. This mimetic compound imitates the natural Smac protein and effectively induces the degradation of IAPs.4,5 Cancer cells escape apoptosis by increasing cIAP1 levels and rely on X-linked inhibitor of apoptosis protein (XIAP) expression for their survival.6,7 Therefore, the antagonism of either cIAP1 or XIAP could potentially lead to heightened responsiveness toward apoptotic triggers. In addition to their direct interaction with and inhibition of caspases, IAPs play a crucial role in numerous cellular processes, such as the transmission of nuclear factor Kappa B signals. Specifically, cIAP1/2 is linked to the tumor necrosis factor receptor 1 (TNFR1) complex and governs the intricate balance between the canonical and noncanonical nuclear factor kappa B signaling pathways.8,9 Earlier investigations conducted in our laboratory have demonstrated that administering substances that sensitize cell death, such as BH3 or Smac mimetics, increases immune cell susceptibility.10-12
CD73 is a transmembrane glycoprotein of type I that is extensively present on the surfaces of the immune system and soft tissue cells.13,14 CD73, functionally speaking, acts as a rate-limiting ecto-5’-nucleotidase (NT5E). This enzyme, responsible for hydrolyzing AMP, plays a crucial role in an ectoenzymatic system that governs the transformation of extracellular adenosine triphosphate into adenosine.15 The significance of CD73 in regulating tumorigenesis, angiogenesis, and metastasis is becoming more recognized, particularly in its contribution to breast cancer (BC) progression, notably through promoting tumor immune evasion.16,17 Consequently, targeting CD73 functions pharmacologically is observed as a highly promising strategy for treating BC.18-20 CD73 is abundantly expressed in various types of cancer as well as by the infiltrating immune cells.21 CD73 demonstrates notable elevation on the plasma membrane of macrophage cells, along with immunosuppressive cells such as myeloid-derived suppressor and Treg cells. Extensive investigation has established a clear association between the increased manifestation of CD73 and the proliferation of cancerous cells, the advancement of metastasis, and the development of fresh blood vessels (angiogenesis).22 In addition, tumor cells with high expression of CD73 are resistant to chemotherapy and immunotherapy. Targeted inhibition of CD73 in cancer cells and its use along with BV6 can be a therapeutic approach with a rational strategy.23 This study evaluated the in vitro effectiveness and synergistic potential of the Smac mimetic BV6 and CD73 inhibition on cancer progression and apoptosis.
Methods
Reagents and Cell Lines
Murine colorectal carcinoma (CT26) and mammary carcinoma (4T1) cell lines were purchased from the Pasteur Institute of Iran (Tehran, Iran). Both cell lines were cultured in RPMI-1640 supplemented with 10% fetal bovine serum (Gibco-Invitrogen, USA), 2% L-glutamine, 100 units/mL penicillin, and 100 mg/mL streptomycin. The cells were maintained at 37 °C in the incubator with 5% CO2 and 95% humidity. Lipofectamine 2000 transfection reagent and 3‐(4,5‐ dimethylthiazol‐2‐yl)‐2,5‐diphenyl tetrazolium bromide (MTT) assay kit were purchased from Sigma-Aldrich (MO, USA). Anti-CD73 and control small interfering ribonucleic acid (siRNAs) were obtained from Santa Cruz (CA, USA).
Cytotoxicity Assay
The MTT assay investigated how BV6 and anti-CD73 siRNA transfection could affect the cells’ viability. Seeded for 24 hours in 96-well plates, the cells were transfected with siRNA molecules (60 pm) and BV6 (27 μM for 4T1 and 31 μM for CT26). The untreated cells were the negative control, while dimethyl sulfoxide (0.2%) was the positive control. Following a 24- or 48-hour incubation period, the cell supernatant was replaced with 100 μL of MTT-containing medium and incubated for four additional hours. Lastly, each well received 100 μL of DMSO for four hours. The supernatant was removed after four hours, and 150 μL of DMSO was added. This mixture was then incubated for 30 minutes. The absorbance was measured at 570 nm and 630 nm for the sample test and reference wavelength, respectively.
Real-time Polymerase Chain Reaction
RNA extraction and cDNA synthesis were performed using RNA extraction and cDNA synthesis kits (BioFACT, Korea). Target gene expression was then measured and amplified using the LightCycler 480 real-time polymerase chain reaction (RT-PCR) system (Roche) and the SYBR Green RT-PCR master mixture (BioFACT). Standard and melting curves were drawn to verify the test’s accuracy. The thermocycling condition of RT-PCR included a one-minute initial denaturation at 95 °C, followed by 40 cycles of amplification (including denaturation at 95 °C for 15 seconds, annealing at 58 °C for 30 seconds, and elongation at 72 °C for 35 seconds). Standard and melting curves were used to verify the test’s accuracy. The data were analyzed using the ΔΔCT method with β-actin as the housekeeping gene.
Apoptosis Assay
The cell Death Detection ELISA kit (Sigma, USA) was used to evaluate the impact of anti-CD73 siRNA and BV6 combination therapy on the apoptosis of cancer cells. In brief, cancer cells (3 × 104) were seeded in 48-well plates and cultured for 24 hours. Subsequently, cells were treated with various therapeutic groups for 48 hours. The cells were then detached from the plate and washed twice (at 1200 rpm for 10 minutes). After one hour of exposure to lysis buffer, the cell pellet was centrifuged once more at 1200 rpm for ten minutes. The cell lysate was utilized for the apoptosis assay using the ELISA kit. The enrichment of mono- and oligo-nucleosomes in the cytoplasm of the apoptotic cells was determined based on the absorbance at 405 nm.
Statistical Analysis
The data were statistically analyzed using GraphPad Prism (version 6) software. The results were reported as means ± standard deviations (SD), and a P-value of less than 0.05 was considered statistically significant.
Results
Silencing CD73 Enhances the Sensitivity of Cancer Cells to BV6-Mediated Cytotoxicity
The results of the MTT assay showed that –although statistically non-significant– silencing CD73 could decrease cancer cell viability to some extent. Meanwhile, BV6 significantly induced cell death in both cell lines. The combined treatment exhibited the greatest cytotoxicity among the therapeutic groups (Figure 1).
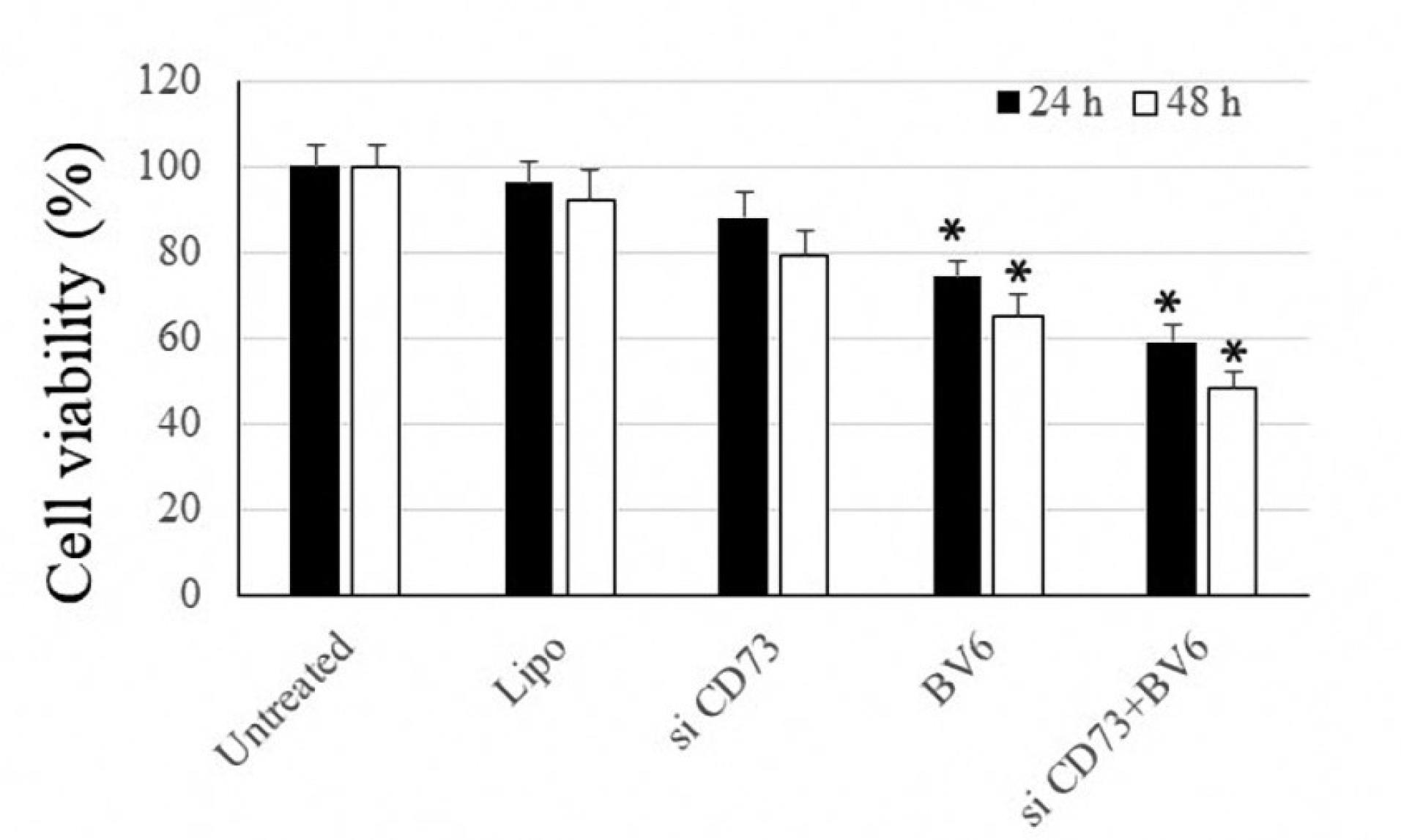
Figure 1.
In Vitro, Cytotoxicity Assessed by MTT Assay for 24 Hours. Note. An asterisk* denotes a P-value less than 0.05. MTT: 3‐(4,5‐ dimethylthiazol‐2‐yl)‐2,5‐diphenyl tetrazolium bromide
.
In Vitro, Cytotoxicity Assessed by MTT Assay for 24 Hours. Note. An asterisk* denotes a P-value less than 0.05. MTT: 3‐(4,5‐ dimethylthiazol‐2‐yl)‐2,5‐diphenyl tetrazolium bromide
CD73 Molecule Expression
The efficacy of siRNA transfection in diminishing CD73 expression was assessed through RT-PCR analysis. As illustrated in Figure 2, the anti-CD73 siRNA notably decreased CD73 expression in both cell lines.
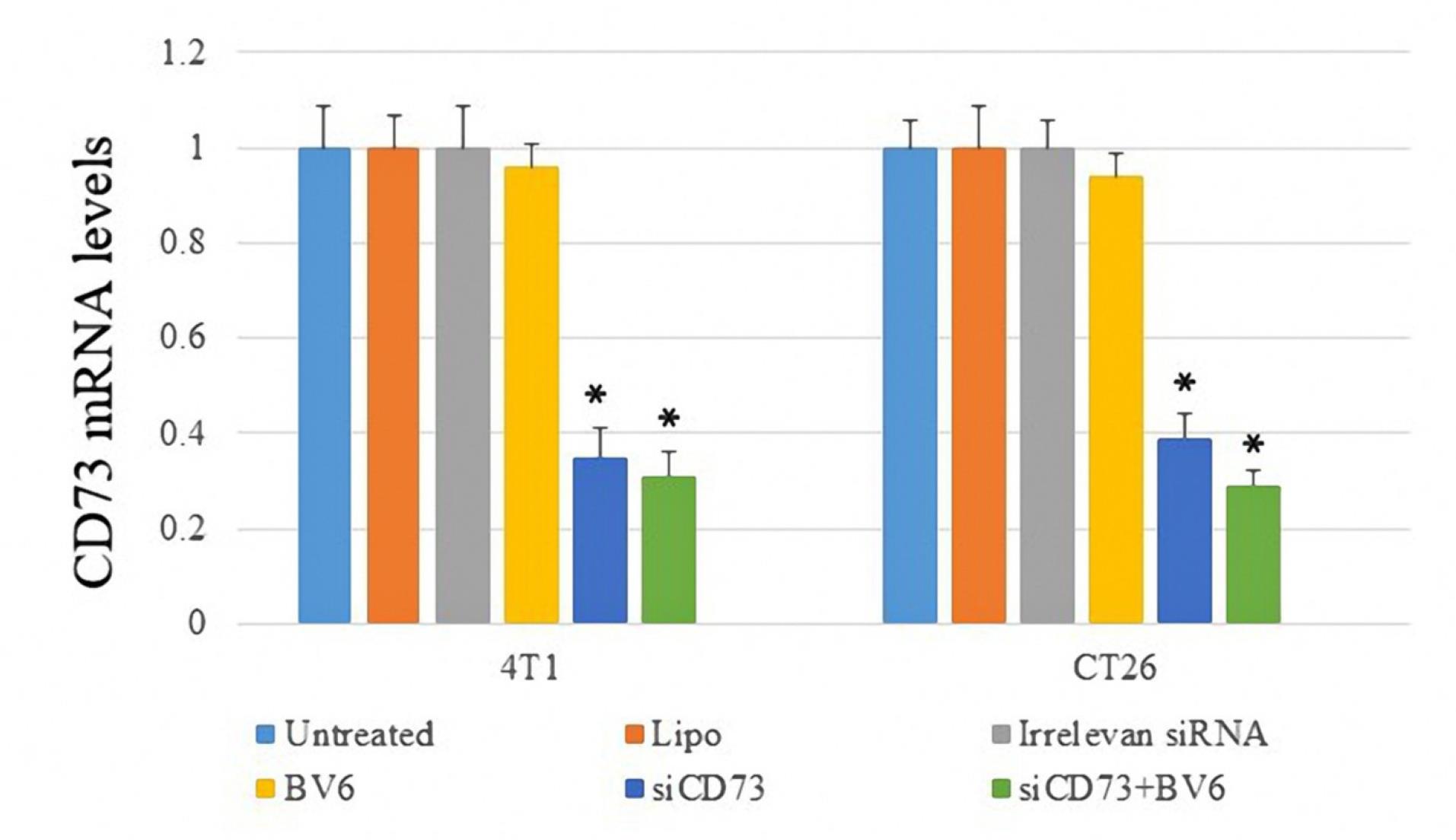
Figure 2.
Real-time Evaluation of CD73 Expression in Both Cell Lines. Note. The asterisk* denotes a P-value less than 0.05
.
Real-time Evaluation of CD73 Expression in Both Cell Lines. Note. The asterisk* denotes a P-value less than 0.05
Apoptosis and Involved Genes
An ELISA-based apoptosis assay was conducted, and the expression of anti-apoptotic BCL-2 and pro-apoptotic BIM genes was recorded. The results demonstrated that although silencing CD73 could not induce significant apoptosis in cancer cells, the treatment of cells with BV6 potently enhanced apoptosis in both cell lines. However, the highest level of apoptosis was recorded through combined treatment (Figure 3a). Moreover, the results showed that treatment with anti-CD73 siRNA and BV6 decreased the Bcl-2 mRNA level, making cells more sensitive to apoptosis (Figure 3b). The combined treatment significantly increased BIM gene expression (Figure 3c).
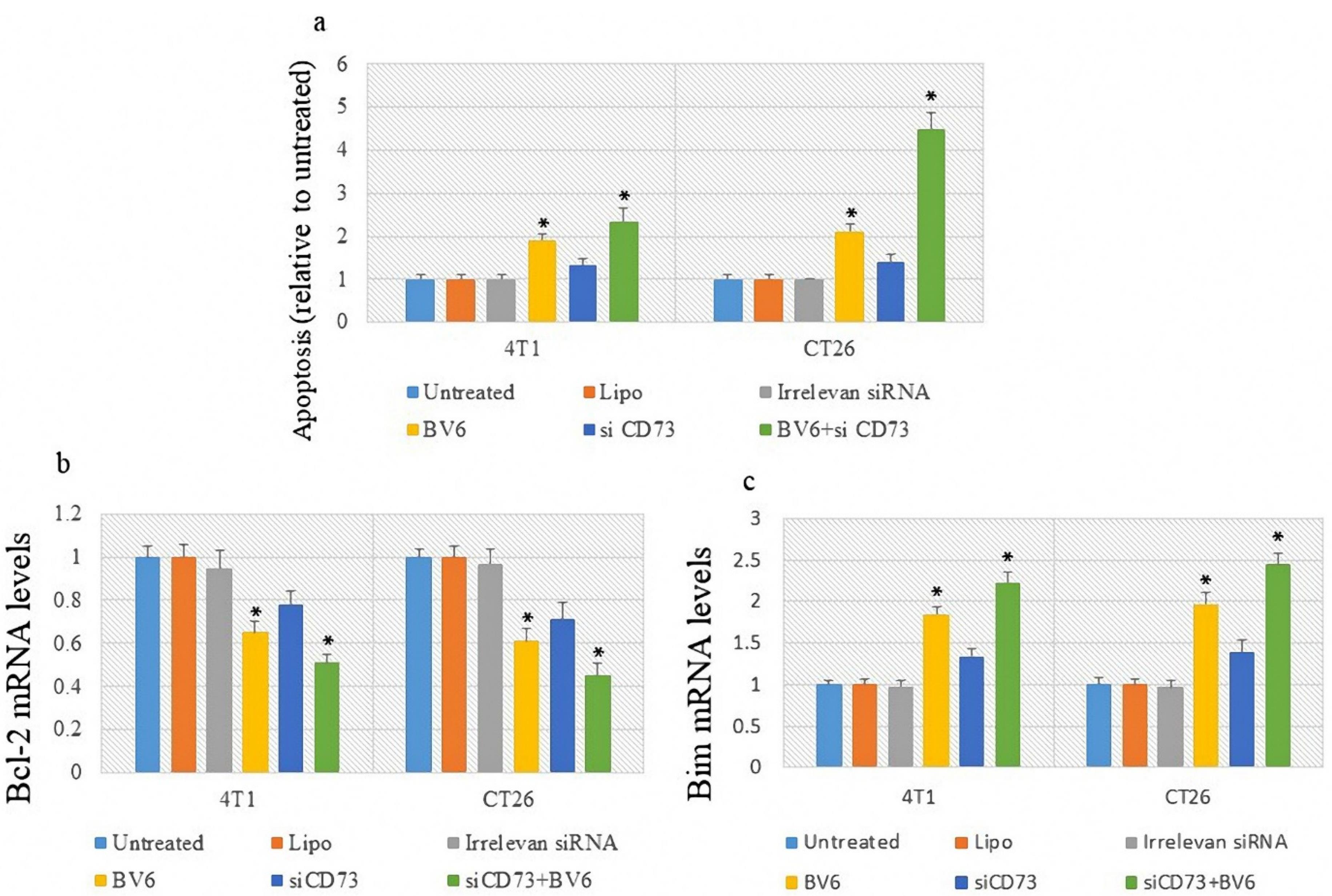
Figure 3.
The Effect of Treatment on Apoptosis and the Expression Level of Apoptosis-Related Genes. Note. ELISA: Enzyme-linked immunosorbent assay; PCR: Polymerase chain reaction. ELISA-based assay was used to evaluate apoptosis (a). Bcl-2 and Bim mRNA expression levels were evaluated using a real-time PCR assay (b and c). Asterisk* represents a P value less than 0.05
.
The Effect of Treatment on Apoptosis and the Expression Level of Apoptosis-Related Genes. Note. ELISA: Enzyme-linked immunosorbent assay; PCR: Polymerase chain reaction. ELISA-based assay was used to evaluate apoptosis (a). Bcl-2 and Bim mRNA expression levels were evaluated using a real-time PCR assay (b and c). Asterisk* represents a P value less than 0.05
Discussion
Cancer has emerged as a significant challenge within the healthcare systems of numerous countries worldwide, posing a formidable threat to public health as the second leading cause of mortality.24 BV6, a bivalent Smac mimetic, has recently been observed to elicit a conformational alteration in the structure of cIAP1. This alteration facilitates the E3 ubiquitin ligase activity within its RING domain, consequently leading to autoubiquitination and subsequent proteasomal degradation.25,26 BV6 specifically targets the inhibitors of apoptosis (IAP), namely, cIAP1, cIAP2, and XIAP, triggering apoptosis in tumor cells.5 BV6, acting alone, triggers apoptosis, as evident by the presence of cleaved caspase-3. Evidence suggests that Smac mimetics can also promote necroptosis in certain contexts, offering an alternative mechanism of cell death.27 This study examined the impact of anti-CD73 siRNA-containing nanoparticles in combination with BV6 on cancer cells. Our findings revealed that the utilization of siRNA-loaded nanoparticles results in a reduction of CD73 gene expression, as well as the expression of genes associated with anti-apoptotic proteins, cell proliferation, angiogenesis, and metastasis.
Fischer et al first demonstrated the impact of BV6 on interleukin (IL)-2-activated expanded natural killer (NK) cells in sensitizing the attack on rhabdomyosarcoma cells. Additionally, they confirmed the transcriptional up-regulation of TNF-related apoptosis-inducing ligand (TRAIL) receptors.11 Recent studies have suggested that BV6 induces cancer cells’ apoptosis by degrading IAP and sensitizing the cells by producing death ligands.28 A study by Anna et al represented that Smac mimetics and proteasome inhibitors are promising therapeutic strategies for primary diffuse large B cell lymphoma, effectively triggering cell death through mitochondrial pathways and enhancing the sensitivity to treatment.29 In another recent study, Smac mimetics have shown the potential to induce apoptosis through noncanonical cell death pathways at elevated concentrations.30 Some investigators sought to inhibit signaling pathways that promote cancer growth, such as both IL-6 and its receptor (glycoprotein 130), and to synergistically reduce cancer progression in vitro.31
The targeting of IAPs via BV6 could potentially serve as an effective strategy to inhibit cancer progression. The findings of a study indicated that BV6 triggers apoptosis in various human cancer cell types, implying that BV6 causes a reduction in cIAP1 and cIAP2 in a dose-dependent manner.28 The radiosensitizing properties of BV6 align with previous findings that the inhibition of IAPs enhances the radiosensitivity of specific types of cancer, such as lung cancer.32-34 Additionally, Checinska et al concluded that the application of a Smac mimetics results in a notable enhancement in cisplatin-induced caspase-3 activity and apoptosis in cancer cells in vitro.35
CD73 has complex and context-dependent antitumor activity. Upregulated CD73 contributes to immune evasion by generating high levels of adenosine, suppressing T cell activity, and promoting tumor growth.36 Consequently, targeting CD73 with specific inhibitors is being explored as a therapeutic strategy to enhance antitumor immunity by counteracting the suppressive effects of adenosine on the immune system.37,38 The coordinated activity of CD73 in conjunction with CD39—another ectonucleotidase—plays a crucial role in regulating the balance between extracellular adenosine triphosphate and adenosine, thereby maintaining overall immune homeostasis.38 Numerous studies have now proved the significant involvement of CD73 in several aspects of immunity and inflammation, including both antitumor immune responses and the evasion of immune surveillance by tumors. By blocking CD73 through antagonistic chemicals or monoclonal antibodies, tumor immunosurveillance could be potentially restored, delaying tumor progress and metastasis through T-cell- and NK-cell-dependent mechanisms, respectively.39 In a study conducted by Gao et al, it was demonstrated that CD73 plays a role in facilitating the growth and movement of cervical cancer cells in humans, regardless of its enzymatic function. In line with the results of our study, their findings indicated that CD73 could potentially enhance the EGFR/Akt and VEGF/Akt pathways, thereby promoting proliferation and migration, independently of its enzymatic activity. These results offer novel perspectives on the regulatory role of CD73 in cancer cells and propose CD73 as a potential target for therapeutic interventions in cervical cancer.40
Yang et al found that the activity of CD73 can be suppressed by tiamulin, leading to the inhibition of BC growth and lung metastasis.41 In another study and a mouse rectal cancer model, Tsukui et al reported that the inhibition of CD73 amplifies the localized and systemic impacts of radiotherapy on coral.42 Our research findings support the notion that suppressing the expression of the CD73 gene is crucial in regulating the spread and growth of rectal cancer cells. Yu et al concluded that CD73 enhances the proliferation of human BC cells via the AKT/GSK-3B/ß-catenin/cyclinD1 signaling cascade,43 which conforms to our research findings on the significance of suppressing CD73 gene expression in regulating BC progression. Zhou et al observed that the upregulation of CD73 enhances the ability of T-47D human BC cells to invade and adhere to the extracellular matrix (ECM). The findings propose that controlled adenosine production, along with changes in EGFR and IL-8 expression resulting from CD73 overexpression, could contribute to the promotion of BC metastasis induced by CD73.44
In another recent study, Jin et al explored a new CD73-targeting antibody-drug conjugate for suppressing lung cancer and enhancing the antitumor immune responses.45 Likewise, Turcotte et al demonstrated that the presence of CD73 is linked to an unfavorable prognosis in high-grade serous ovarian cancer.46 Miyazaki et al have presented evidence suggesting that the presence of tumor ECM metalloproteinase inducer and heightened stromal CD73 levels are associated with a poor prognosis in cases of external auditory canal carcinoma.47 All these results corroborate our study findings, highlighting the significance of CD73 in tumor immune responses.
Conclusion
Data obtained from all the stages of our study confirm the tumor inhibitory effects of the combined targeting of CD73 and treatment of BV6. In this study, the researchers first targeted this synergistic cycle and used this novel therapeutic combination. The development of a powerful nanocarrier system with conjugated trimethyl chitosan-alginate for this compound is another strength of this study, which increases the effectiveness of the treatment. However, future in vivo studies are required to prove the effectiveness of this therapeutic approach.
Ethics statement
This study was ethically approved by Tabriz University of Medical Sciences (Ethical number: IR.TBZMED.VCR.REC.1399.453).
Conflict of interests declaration
The researchers have no conflict of interests to declare.
Acknowledgments
We would like to thank Tabriz University of Medical Sciences for the financial support of this study (grant number: 66443).
Data availability statement
The data related to the study are available upon request from the corresponding author upon reasonable request.
Author contributions
Conceptualization: Farhad Jadidi-Niaragh, Sakineh Hajebrahimi, Sanam Nami.
Data curation: Maryam Farajzadeh, Mohammad-Salar Hosseini, Mehran Mozaffari.
Formal analysis: Maryam Farajzadeh, Mohammad-Salar Hosseini
Funding acquisition: Sanam Nami.
Investigation: Maryam Farajzadeh, Mohammad-Salar Hosseini, Farzaneh Sadat Eshaghi, Fatemeh Alian.
Methodology: Maryam Farajzadeh, Mohammad-Salar Hosseini, Farzaneh Sadat Eshaghi, Fatemeh Alian.
Project administration: Farhad Jadidi-Niaragh.
Resources: Farhad Jadidi-Niaragh.
Software: Maryam Farajzadeh, Mohammad-Salar Hosseini.
Supervision: Farhad Jadidi-Niaragh.
Validation: Sakineh Hajebrahimi, Sanam Nami, Farhad Jadidi-Niaragh.
Visualization: Sakineh Hajebrahimi, Sanam Nami, Farhad Jadidi-Niaragh.
Writing–original draft: Maryam Farajzadeh, Mohammad-Salar Hosseini, Farhad Jadidi-Niaragh.
Writing–review & editing: Maryam Farajzadeh, Mohammad-Salar Hosseini, Farhad Jadidi-Niaragh.
References
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024; 74(3):229-63. doi: 10.3322/caac.21834 [Crossref] [ Google Scholar]
- Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell 2009; 15(2):79-80. doi: 10.1016/j.ccr.2009.01.009 [Crossref] [ Google Scholar]
- Abou-Shousha S, Moaaz M, Sheta M, Motawea MA. An approach to breast cancer immunotherapy: the apoptotic activity of recombinant anti-interleukin-6 monoclonal antibodies in intact tumour microenvironment of breast carcinoma. Scand J Immunol 2016; 83(6):427-37. doi: 10.1111/sji.12426 [Crossref] [ Google Scholar]
- Sun H, Nikolovska-Coleska Z, Lu J, Meagher JL, Yang CY, Qiu S. Design, synthesis, and characterization of a potent, nonpeptide, cell-permeable, bivalent SMAC mimetic that concurrently targets both the BIR2 and BIR3 domains in XIAP. J Am Chem Soc 2007; 129(49):15279-94. doi: 10.1021/ja074725f [Crossref] [ Google Scholar]
- Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell 2007; 131(4):669-81. doi: 10.1016/j.cell.2007.10.030 [Crossref] [ Google Scholar]
- Faye MD, Beug ST, Graber TE, Earl N, Xiang X, Wild B. IGF2BP1 controls cell death and drug resistance in rhabdomyosarcomas by regulating translation of cIAP1. Oncogene 2015; 34(12):1532-41. doi: 10.1038/onc.2014.90 [Crossref] [ Google Scholar]
- Holt SV, Brookes KE, Dive C, Makin GW. Down-regulation of XIAP by AEG35156 in paediatric tumour cells induces apoptosis and sensitises cells to cytotoxic agents. Oncol Rep 2011; 25(4):1177-81. doi: 10.3892/or.2011.1167 [Crossref] [ Google Scholar]
- Li J, Yin Q, Wu H. Structural basis of signal transduction in the TNF receptor superfamily. Adv Immunol 2013; 119:135-53. doi: 10.1016/b978-0-12-407707-2.00005-9 [Crossref] [ Google Scholar]
- Lee S, Challa-Malladi M, Bratton SB, Wright CW. Nuclear factor-κB-inducing kinase (NIK) contains an amino-terminal inhibitor of apoptosis (IAP)-binding motif (IBM) that potentiates NIK degradation by cellular IAP1 (c-IAP1). J Biol Chem 2014; 289(44):30680-9. doi: 10.1074/jbc.M114.587808 [Crossref] [ Google Scholar]
- Särchen V, Shanmugalingam S, Kehr S, Reindl LM, Greze V, Wiedemann S. Pediatric multicellular tumor spheroid models illustrate a therapeutic potential by combining BH3 mimetics with natural killer (NK) cell-based immunotherapy. Cell Death Discov 2022; 8(1):11. doi: 10.1038/s41420-021-00812-6 [Crossref] [ Google Scholar]
- Fischer K, Tognarelli S, Roesler S, Boedicker C, Schubert R, Steinle A. The SMAC mimetic BV6 improves NK cell-mediated killing of rhabdomyosarcoma cells by simultaneously targeting tumor and effector cells. Front Immunol 2017; 8:202. doi: 10.3389/fimmu.2017.00202 [Crossref] [ Google Scholar]
- Rettinger E, Glatthaar A, Abhari BA, Oelsner S, Pfirrmann V, Huenecke S. SMAC mimetic BV6 enables sensitization of resistant tumor cells but also affects cytokine-induced killer (CIK) cells: a potential challenge for combination therapy. Front Pediatr 2014; 2:75. doi: 10.3389/fped.2014.00075 [Crossref] [ Google Scholar]
- Alcedo KP, Bowser JL, Snider NT. The elegant complexity of mammalian ecto-5’-nucleotidase (CD73). Trends Cell Biol 2021; 31(10):829-42. doi: 10.1016/j.tcb.2021.05.008 [Crossref] [ Google Scholar]
- Borea PA, Gessi S, Merighi S, Varani K. Adenosine as a multi-signalling guardian angel in human diseases: when, where and how does it exert its protective effects?. Trends Pharmacol Sci 2016; 37(6):419-34. doi: 10.1016/j.tips.2016.02.006 [Crossref] [ Google Scholar]
- Antonioli L, Pacher P, Vizi ES, Haskó G. CD39 and CD73 in immunity and inflammation. Trends Mol Med 2013; 19(6):355-67. doi: 10.1016/j.molmed.2013.03.005 [Crossref] [ Google Scholar]
- Jadidi-Niaragh F. Potential of CD73 as a target for cancer immunotherapy. Immunotherapy 2019; 11(16):1353-5. doi: 10.2217/imt-2019-0147 [Crossref] [ Google Scholar]
- Jadidi-Niaragh F, Atyabi F, Rastegari A, Mollarazi E, Kiani M, Razavi A. Downregulation of CD73 in 4T1 breast cancer cells through siRNA-loaded chitosan-lactate nanoparticles. Tumour Biol 2016; 37(6):8403-12. doi: 10.1007/s13277-015-4732-0 [Crossref] [ Google Scholar]
- Boison D, Yegutkin GG. Adenosine metabolism: emerging concepts for cancer therapy. Cancer Cell 2019; 36(6):582-96. doi: 10.1016/j.ccell.2019.10.007 [Crossref] [ Google Scholar]
- Antonioli L, Novitskiy SV, Sachsenmeier KF, Fornai M, Blandizzi C, Haskó G. Switching off CD73: a way to boost the activity of conventional and targeted antineoplastic therapies. Drug Discov Today 2017; 22(11):1686-96. doi: 10.1016/j.drudis.2017.06.005 [Crossref] [ Google Scholar]
- Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci U S A 2010; 107(4):1547-52. doi: 10.1073/pnas.0908801107 [Crossref] [ Google Scholar]
- Tripathi A, Lin E, Xie W, Flaifel A, Steinharter JA, Stern Gatof EN. Prognostic significance and immune correlates of CD73 expression in renal cell carcinoma. J Immunother Cancer 2020; 8(2):e001467. doi: 10.1136/jitc-2020-001467 [Crossref] [ Google Scholar]
- Kapadia CH, Perry JL, Tian S, Luft JC, DeSimone JM. Nanoparticulate immunotherapy for cancer. J Control Release 2015; 219:167-80. doi: 10.1016/j.jconrel.2015.09.062 [Crossref] [ Google Scholar]
- Sadeghi M, Yousefi Bostanabad M, Abedi O, Hosseinpour Feizi AA, Movasaghpour Akbari AA, Jadidi-Niaragh F. Blockade of CD73 increases the cytotoxic effects of fludarabine in chronic lymphocytic leukemia. ImmunoAnalysis 2022; 2(1):11. doi: 10.34172/ia.2022.11 [Crossref] [ Google Scholar]
- Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer: review and recommendations. J Clin Oncol 2012; 30(11):1160-77. doi: 10.1200/jco.2011.39.5509 [Crossref] [ Google Scholar]
- Dueber EC, Schoeffler AJ, Lingel A, Elliott JM, Fedorova AV, Giannetti AM. Antagonists induce a conformational change in cIAP1 that promotes autoubiquitination. Science 2011; 334(6054):376-80. doi: 10.1126/science.1207862 [Crossref] [ Google Scholar]
- Feltham R, Bettjeman B, Budhidarmo R, Mace PD, Shirley S, Condon SM. SMAC mimetics activate the E3 ligase activity of cIAP1 protein by promoting RING domain dimerization. J Biol Chem 2011; 286(19):17015-28. doi: 10.1074/jbc.M111.222919 [Crossref] [ Google Scholar]
- Safferthal C, Rohde K, Fulda S. Therapeutic targeting of necroptosis by SMAC mimetic bypasses apoptosis resistance in acute myeloid leukemia cells. Oncogene 2017; 36(11):1487-502. doi: 10.1038/onc.2016.310 [Crossref] [ Google Scholar]
- El-Mesery M, Shaker ME, Elgaml A. The SMAC mimetic BV6 induces cell death and sensitizes different cell lines to TNF-α and TRAIL-induced apoptosis. Exp Biol Med (Maywood) 2016; 241(18):2015-22. doi: 10.1177/1535370216661779 [Crossref] [ Google Scholar]
- Dietz A, Dalda N, Zielke S, Dittmann J, van Wijk SJ, Vogler M. Proteasome inhibitors and SMAC mimetics cooperate to induce cell death in diffuse large B-cell lymphoma by stabilizing NOXA and triggering mitochondrial apoptosis. Int J Cancer 2020; 147(5):1485-98. doi: 10.1002/ijc.32976 [Crossref] [ Google Scholar]
- Miles MA, Caruso S, Baxter AA, Poon IKH, Hawkins CJ. SMAC mimetics can provoke lytic cell death that is neither apoptotic nor necroptotic. Apoptosis 2020; 25(7-8):500-18. doi: 10.1007/s10495-020-01610-8 [Crossref] [ Google Scholar]
- Khodayari P, Khodakarami A, Hassannia H, Ghalamfarsa G, Hojjat-Farsangi M, Hashemi V. Silencing interleukin-6 and glycoprotein 130 suppresses growth and induces apoptosis in cancer cells. Int J Drug Res Clin 2023; 1(1):e8. doi: 10.34172/ijdrc.2023.e8 [Crossref] [ Google Scholar]
- Huerta S, Gao X, Livingston EH, Kapur P, Sun H, Anthony T. In vitro and in vivo radiosensitization of colorectal cancer HT-29 cells by the SMAC mimetic JP-1201. Surgery 2010; 148(2):346-53. doi: 10.1016/j.surg.2010.05.006 [Crossref] [ Google Scholar]
- Dai Y, Liu M, Tang W, DeSano J, Burstein E, Davis M. Molecularly targeted radiosensitization of human prostate cancer by modulating inhibitor of apoptosis. Clin Cancer Res 2008; 14(23):7701-10. doi: 10.1158/1078-0432.Ccr-08-0188 [Crossref] [ Google Scholar]
- Iwasa T, Okamoto I, Suzuki M, Nakahara T, Yamanaka K, Hatashita E. Radiosensitizing effect of YM155, a novel small-molecule survivin suppressant, in non-small cell lung cancer cell lines. Clin Cancer Res 2008; 14(20):6496-504. doi: 10.1158/1078-0432.Ccr-08-0468 [Crossref] [ Google Scholar]
- Checinska A, Hoogeland BS, Rodriguez JA, Giaccone G, Kruyt FA. Role of XIAP in inhibiting cisplatin-induced caspase activation in non-small cell lung cancer cells: a small molecule SMAC mimic sensitizes for chemotherapy-induced apoptosis by enhancing caspase-3 activation. Exp Cell Res 2007; 313(6):1215-24. doi: 10.1016/j.yexcr.2006.12.011 [Crossref] [ Google Scholar]
- Bach N, Winzer R, Tolosa E, Fiedler W, Brauneck F. The clinical significance of CD73 in cancer. Int J Mol Sci 2023; 24(14):11759. doi: 10.3390/ijms241411759 [Crossref] [ Google Scholar]
- Zhang M, Dai X, Xiang Y, Xie L, Sun M, Shi J. Advances in CD73 inhibitors for immunotherapy: antibodies, synthetic small molecule compounds, and natural compounds. Eur J Med Chem 2023; 258:115546. doi: 10.1016/j.ejmech.2023.115546 [Crossref] [ Google Scholar]
- Kaplinsky N, Williams K, Watkins D, Adams M, Stanbery L, Nemunaitis J. Regulatory role of CD39 and CD73 in tumor immunity. Future Oncol 2024; 20(19):1367-80. doi: 10.2217/fon-2023-0871 [Crossref] [ Google Scholar]
- Allard B, Turcotte M, Stagg J. Targeting CD73 and downstream adenosine receptor signaling in triple-negative breast cancer. Expert Opin Ther Targets 2014; 18(8):863-81. doi: 10.1517/14728222.2014.915315 [Crossref] [ Google Scholar]
- Gao ZW, Wang HP, Lin F, Wang X, Long M, Zhang HZ. CD73 promotes proliferation and migration of human cervical cancer cells independent of its enzyme activity. BMC Cancer 2017; 17(1):135. doi: 10.1186/s12885-017-3128-5 [Crossref] [ Google Scholar]
- Yang X, Pei S, Wang H, Jin Y, Yu F, Zhou B. Tiamulin inhibits breast cancer growth and pulmonary metastasis by decreasing the activity of CD73. BMC Cancer 2017; 17(1):255. doi: 10.1186/s12885-017-3250-4 [Crossref] [ Google Scholar]
- Tsukui H, Horie H, Koinuma K, Ohzawa H, Sakuma Y, Hosoya Y. CD73 blockade enhances the local and abscopal effects of radiotherapy in a murine rectal cancer model. BMC Cancer 2020; 20(1):411. doi: 10.1186/s12885-020-06893-3 [Crossref] [ Google Scholar]
- Yu J, Wang X, Lu Q, Wang J, Li L, Liao X. Extracellular 5’-nucleotidase (CD73) promotes human breast cancer cells growth through AKT/GSK-3β/β-catenin/cyclinD1 signaling pathway. Int J Cancer 2018; 142(5):959-67. doi: 10.1002/ijc.31112 [Crossref] [ Google Scholar]
- Zhou P, Zhi X, Zhou T, Chen S, Li X, Wang L. Overexpression of ecto-5’-nucleotidase (CD73) promotes T-47D human breast cancer cells invasion and adhesion to extracellular matrix. Cancer Biol Ther 2007; 6(3):426-31. doi: 10.4161/cbt.6.3.3762 [Crossref] [ Google Scholar]
- Jin R, Liu L, Xing Y, Meng T, Ma L, Pei J. Dual mechanisms of novel CD73-targeted antibody and antibody-drug conjugate in inhibiting lung tumor growth and promoting antitumor immune-effector function. Mol Cancer Ther 2020; 19(11):2340-52. doi: 10.1158/1535-7163.Mct-20-0076 [Crossref] [ Google Scholar]
- Turcotte M, Spring K, Pommey S, Chouinard G, Cousineau I, George J. CD73 is associated with poor prognosis in high-grade serous ovarian cancer. Cancer Res 2015; 75(21):4494-503. doi: 10.1158/0008-5472.Can-14-3569 [Crossref] [ Google Scholar]
- Miyazaki M, Aoki M, Okado Y, Koga K, Hamasaki M, Nakagawa T. Highly expressed tumoral EMMPRIN and stromal CD73 predict a poor prognosis for external auditory canal carcinoma. Cancer Sci 2020; 111(8):3045-56. doi: 10.1111/cas.14508 [Crossref] [ Google Scholar]