Int J Drug Res Clin. 2:e19.
doi: 10.34172/ijdrc.2024.e19
Original Article
Combined Blockade of Interleukin-6 and β-Catenin Inhibits Breast and Colon Tumor Growth In Vitro
Maryam Farajzadeh 1  , Mohammad-Salar Hosseini 2, Farzaneh Eshaghi 1, Samira Mohebbi 3, Mohammad Hojjat-Farsangi 4, Farhad Jadidi-Niaragh 1, 2, 5, *
, Mohammad-Salar Hosseini 2, Farzaneh Eshaghi 1, Samira Mohebbi 3, Mohammad Hojjat-Farsangi 4, Farhad Jadidi-Niaragh 1, 2, 5, * 
Author information:
1Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2Research Center for Integrative Medicine in Aging, Aging Research Institute, Tabriz University of Medical Sciences, Tabriz, Iran
3Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran
4Department of Oncology-Pathology, Karolinska Institute, Bioclinicum, Stockholm, Sweden
5Department of Immunology, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Background:
As the primary regulator of tumor activities, the tumor microenvironment contains several factors contributing to tumor progression and metastasis. Interleukin 6 (IL-6) and β-catenin are two key factors in the tumor microenvironment, which are effective in tumor progression in primary studies. This study aimed to evaluate the impact of IL-6 and β-catenin blockade on tumor growth and progression in vitro.
Methods:
In this study, small interfering RNA (siRNA) technology was used to target IL-6 and β-catenin using two murine cell lines, 4T1 (breast cancer) and CT26 (colon cancer). The target gene expression was analyzed by applying quantitative real-time polymerase chain reaction assays. The MTT assay was used to measure how a treatment could affect the cells’ survival ability. All data were analyzed by GraphPad Prism 9, and P-values less than 0.05 were considered significant.
Results:
Both cell lines demonstrated downregulation in the expression patterns of genes involved in apoptosis, metastasis, proliferation, and angiogenesis. Both cell lines showed significantly decreased IL-6 secretion following IL-6 targeting. Moreover, simultaneous targeting of IL-6 and β-catenin factors significantly reduced the survival of cancer cells.
Conclusion:
These results revealed that targeting β-catenin and IL-6 simultaneously may be a useful treatment strategy for colon and breast cancers. Further investigations are required to determine the effectiveness of these interventions and adjust their safety.
Keywords: Beta-catenin, Cancer immunotherapy, Combination therapy, IL-6
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
This study supported by Tabriz University of Medical Sciences (grant numbers: 69958 and 73516).
Please cite this article as follows: Farajzadeh M, Hosseini MS, Eshaghi F, Mohebbi S, Hojjat-Farsangi M, Jadidi-Niaragh F. Combined blockade of interleukin-6 and β-catenin inhibits breast and colon tumor growth in vitro. Int J Drug Res Clin. 2024; 2: e19. doi: 10.34172/ijdrc.2024.e19
Introduction
Modern therapeutic strategies target tumor microenvironment (TME) inhibitory pathways.1 So far, several components of the immune system have been studied to promote inhibitory TME, encompassing, but not restricted to, regulatory T cells, myeloid-derived suppressor cells, natural killer cells, and signaling pathways such as wingless-related integration site (Wnt)/β-catenin and phosphatidylinositol-3 kinase/protein kinase B.2,3
TME comprises elements that regulate the sustenance and operation of cancer cells.4 Tumoral and stromal cells release various signaling molecules, facilitating the recruitment of immune cells and their activities in the TME. Consequently, the recruited cells can promote a pro-inflammatory microenvironment by producing cytokines from the interleukin 6 (IL-6) family.5,6 Hence, persistent inflammation contributes to the adaptability of the TME and establishes a microenvironment that promotes tumor growth, immune system suppression, and the advancement of cancer.7 IL-6 plays a crucial role as a cytokine in transforming CD4 + T into Th17 cells, and IL-6-targeting therapeutic agents have been authorized as efficacious treatments for these conditions.8,9 Approaches aimed at blocking IL-6 signaling through using antibodies and small molecules targeting IL-6, the IL-6 receptor, or downstream signaling molecules such as Janus kinases (JAKs) have progressed to clinical trials and demonstrated efficacy.10 These strategies have also been proven to be effective in managing life-threatening cytokine storms linked to CAR-T cell therapy in cancer patients.11,12
β-Catenin, a versatile protein, regulates physiological functions.13 Its abnormal upregulation is linked with the development of many diseases, including cancer.14 β-catenin has a crucial role in cancer advancement, aggression, invasion, and metastasis by controlling the transcriptional activity of various oncogenes and tumor suppressor genes.15 Due to its critical role in multiple malignant processes, including cancer stemness and metastasis, the β-catenin signaling system has been extensively studied, making it a prospective target for therapeutic interventions.16 Despite the significance of its role in carcinogenesis, the progress in developing therapeutic strategies to target the β-catenin molecule remains in its early stages.17 Several approaches have been devised thus far to target β-catenin, including small molecules, antibodies, and peptides.18 The stability and transcriptional activity of β-catenin are influenced by numerous binding partners, which play an essential role in regulating its function. A comprehensive understanding of these regulatory mechanisms is essential in identifying potential therapeutic targets for combating Wnt/β-catenin-associated cancers.16 This investigation aimed to address the issue of cancer resistance by manipulating the immune TME through the targeted inhibition of β-catenin and the blockade of IL-6 cytokine signaling.
Methods
Materials
Control, β-catenin, and anti-murine IL-6-targeting small interfering RNA (siRNA) molecules were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). In addition, the Lipofectamine RNAiMAX Transfection Reagent was obtained from Thermo Fisher Scientific in Waltham, Massachusetts, in the United States. Takara (Shiga, Japan) provided the real-time polymerase chain reaction (qPCR) master mix, while BioFact (Daejeon, South Korea) provided the RNA extraction and complementary DNA (cDNA) synthesis kits. The 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT)-based in Vitro Toxicology Assay Kit was bought from Sigma-Aldrich Company (St. Louis, Missouri, United States). Finally, mouse enzyme-linked immunosorbent assay (ELISA) kits were purchased from eBioScience (San Diego, CA, USA).
Cell Lines and Culture
Murine colorectal carcinoma (CT26) and mammary carcinoma (4T1) cell lines were obtained from the National Cell Bank of Iran (Pasteur Institute of Iran, Tehran, Iran) and cultured in the RPMI-1640 medium supplemented with 10% fetal bovine serum supplemented with 100 unit/mL penicillin and 100 mg/mL streptomycin (Gibco, Grand Island, NY, USA). Standard procedures were followed during the 37 °C incubation of the cancer cells with a 5% concentration of CO2. The culture medium was replaced every two days.
Cell Transfection With Small Interfering RNA
Cells (1 × 104 cells/well) were cultured in 96-well plates for 24 hours. Following the manufacturer’s guidelines, siRNAs targeting β-catenin and anti-IL-6 were transfected into the cells (60 pm) using Lipofectamine. Naked siRNAs were used as a control. The supernatant of each well was then collected and utilized to evaluate IL-6 levels.
Gene Expression Analysis
RNA was isolated using RNA extraction kits (BioFact, South Korea). cDNA was generated from the isolated RNA by utilizing cDNA synthesis kits (BioFact, South Korea). The RT-PCR assay was performed utilizing a 6000 Gene-Rotor Corbett along with SYBR Green Master Mix to enhance the amplification of target gene expression. The thermocycling conditions for RT-PCR included an initial denaturation step at 95 °C for 1 minute. Subsequently, 40 amplification cycles were conducted, with each cycle comprising a denaturation step at 95 °C for 15 seconds, an annealing phase at 58 °C for 30 seconds, and an elongation period at 72 °C for 35 seconds. The assay’s accuracy was verified by creating standard and melting curves. Additionally, the Livak method (2-ΔΔCT) was utilized to determine the relative transcript levels for each target gene, with the transcript level of β-actin serving as the housekeeping gene. Table 1 presents the utilized primer sequences.19,20
Table 1.
Sequences Employed in This Research
|
Gene
|
Forward
|
Reverse
|
| β-actin |
5′- GGTCATCACTATTGGCAACG -3′ |
5′- ACGGATGTCAACGTCACACT -3′ |
| IL-6 |
5′- ATCCAGTTGCCTTCTTGGGACTGA -3′ |
5′- TAAGCCTCCGACTTGTGAAGTGGT -3′ |
| β-catenin |
5′-ACAGCACCTTCAGCACTCT-3′ |
5′-AAGTTCTTGGCTATTACGACA-3′ |
| MMP2 |
5′- TGTGTCTTCCCCTTCACTTT -3′ |
5′- GATCTGAGCGATGCCATCAA -3′ |
| MMP9 |
5′- ACACGACATCTTCCAGTACC -3′ |
5′- CAGGAGGTCGTAGGTCACGTAGC -3′ |
| VEGF |
5′- CTGGATATGTTTGACTGCTGTGGA -3′ |
5′- GTTTCTGGAAGTGAGCCAATGTG -3′ |
| BCL2 |
5′- GGCTGGGGATGACTTCTCTC -3′ |
5′- ACAATCCTCCCCCAGTTCAC -3′ |
| BIM |
5′- GAGATACGGATTGCACAGGA -3′ |
5′- TCAGCCTCGCGGTAATCATT -3′ |
| FGF |
5′- CCCACCAGGCCACTTCAA -3′ |
5′- GATGGATGCGCAGGAAGAA -3′ |
Note. Bcl-2: B-cell lymphoma 2; BIM: Bcl-2 interacting mediator; FGF: Fibroblast growth factors; IL: Interleukin; MMPs: Matrix metalloproteinases; VEGF: Vascular endothelial growth factor; FGF: Fibroblast growth factor.
3-(4,5-Dimethyl-Thiazol-2-yl)-2,5-Diphenyl-Tetrazoliumbromide Assay
An MTT assay was employed to measure in vitro cytotoxicity.21 Following a 24-hour incubation, the cells were treated with the treatment groups for up to 48 hours. Further, the serum-free medium and a 10 µL MTT solution were added to the cultured cells’ supernatant and incubated for an additional 4 hours. Finally, the culture medium was replaced with 100 μL dimethyl sulfoxide and incubated for 30 minutes. The viable cells’ ratio was assessed by analyzing the optical density at 570 nm and 630 nm.
Enzyme-Linked Immunosorbent Assay
The ELISA assay was utilized to investigate the effects of siRNA transfection on the secretion levels of IL-6 and β-catenin.22 In brief, cancer cells were cultured in 96-well plates for a duration of 24 hours. The cells were then treated with various therapeutic groups for 48 hours. Next, each well’s supernatant was gathered and used to evaluate IL-6 and β-catenin levels using the ELISA kit (eBioScience).
Statistical Analysis
The statistical analysis was conducted by utilizing GraphPad Prism 9.0. Using a one-way analysis of variance, the results were expressed as means ± standard deviations (SD). The importance level was established as a P value < 0.05. All tests were performed in triplicate and repeated at least twice.
Results
Downregulation of Interleukin 6 and β-Catenin Reduced Cancer Cell Viability
Following the MTT assay, IL-6 and β-catenin showed a reduced viability in either mono- or combined blockade in both cell lines. Combined treatment had the highest efficacy in reducing cell survival compared to other treatments (Figure 1).
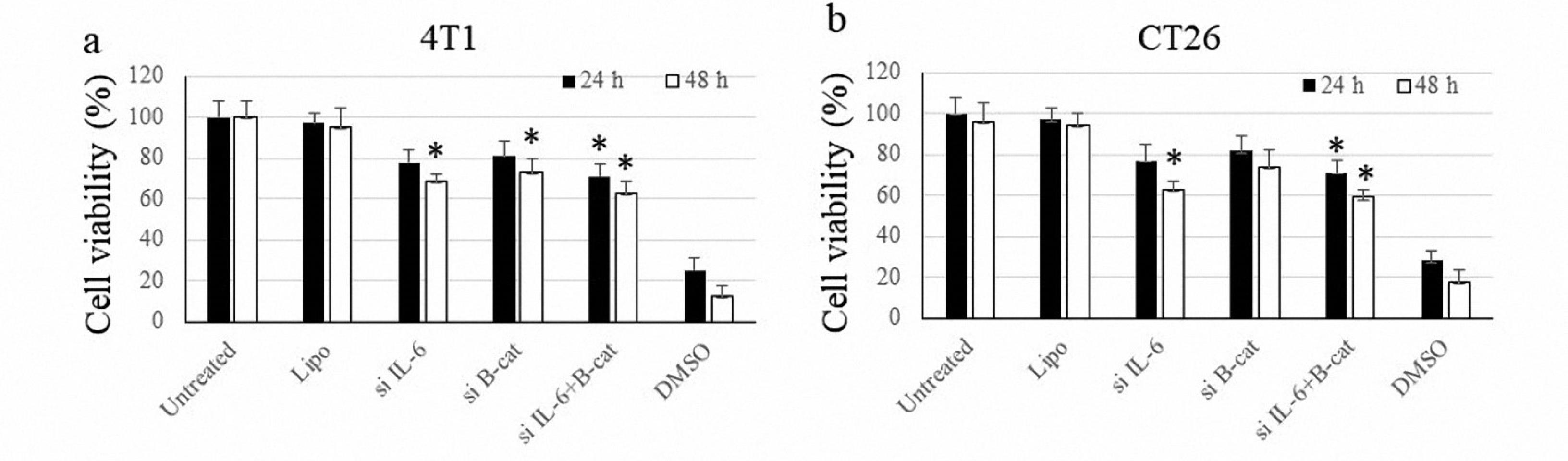
Figure 1.
Cancer Cells’ Viability After Transfection With siRNA Molecules Against IL-6 and β-Catenin in 4T1 and CT26 Cells After 24 and 48 Hours of Incubation. Note. siRNA: Small interfering RNA; IL: Interleukin. The symbol * indicates P value ≤ 0.05
.
Cancer Cells’ Viability After Transfection With siRNA Molecules Against IL-6 and β-Catenin in 4T1 and CT26 Cells After 24 and 48 Hours of Incubation. Note. siRNA: Small interfering RNA; IL: Interleukin. The symbol * indicates P value ≤ 0.05
Expression of β-Catenin and IL-6 Suppressed by Transfecting Cancer Cells With siRNA Molecules
The PCR method was utilized to investigate the efficacy of siRNA transfection in expressing IL-6 and β-catenin within cancer cells. As shown in Figure 2, the IL-6-targeting siRNA could effectively reduce IL-6 expression in both cell lines. Cells treated with siRNA targeting β-catenin also showed consistent results. Remarkably, inhibiting each of the IL-6 and β-catenin factors had a suppressing effect on the expression of the other factor, although this decrease was not statistically significant. Similar to the mRNA level, the examination of the treatment’s impact on IL-6 protein secretion levels revealed a marked reduction in the secreted protein.
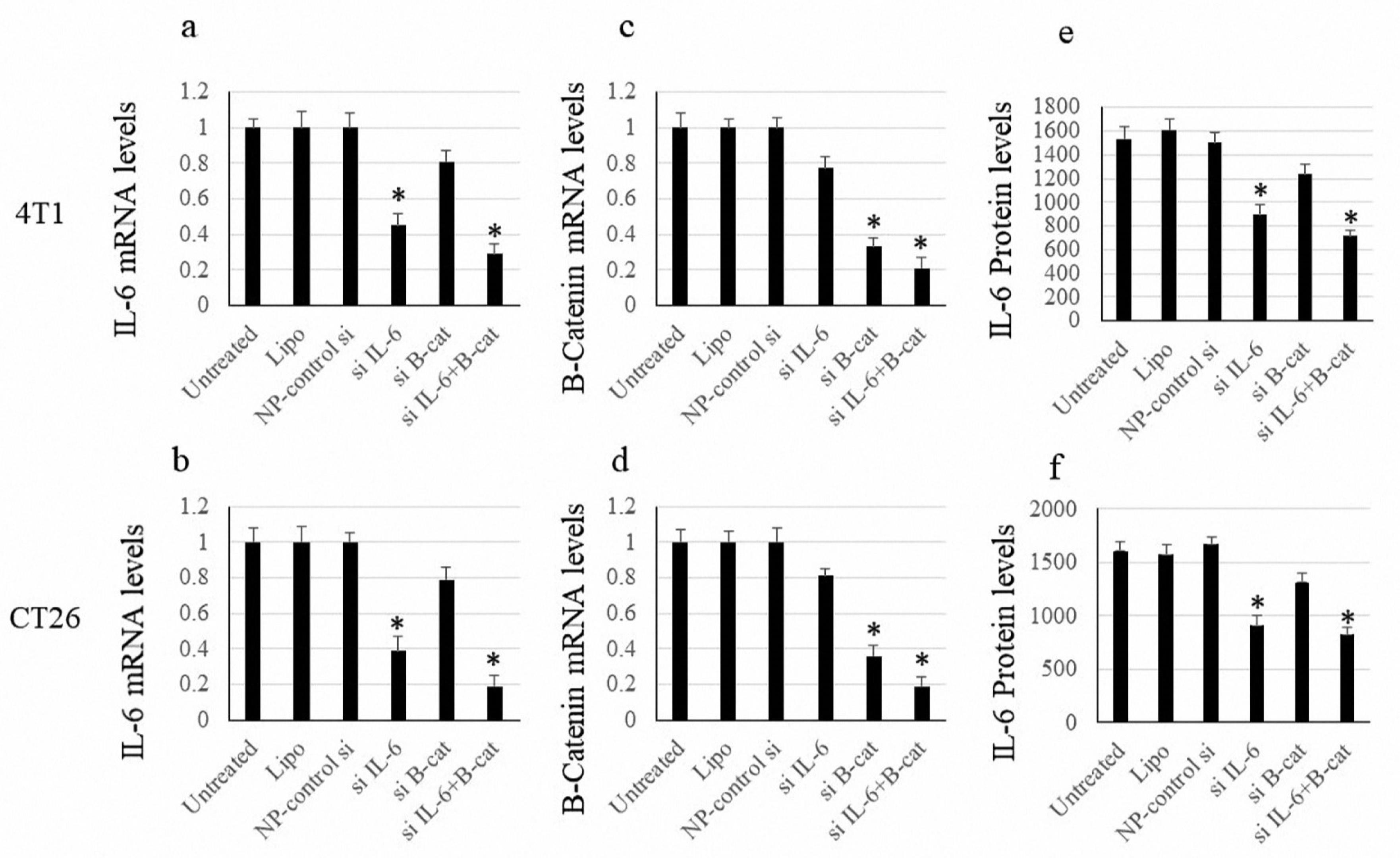
Figure 2.
Expression Levels of IL-6 and β-Catenin. Note. IL: Interleukin; SD: Standard deviation. The data are expressed as means ± SDs of IL-6 and β-catenin levels. Vertical axes demonstrate the fold changes. The symbols * and ** indicate P values < 0.05 and < 0.01, respectively
.
Expression Levels of IL-6 and β-Catenin. Note. IL: Interleukin; SD: Standard deviation. The data are expressed as means ± SDs of IL-6 and β-catenin levels. Vertical axes demonstrate the fold changes. The symbols * and ** indicate P values < 0.05 and < 0.01, respectively
Silencing Interleukin 6 and β-Catenin Regulates the Expression of Genes Linked to Apoptosis
To examine how a treatment affects the expression of apoptosis-related genes, pro-apoptotic Bax and anti-apoptotic Bcl-2 factors were examined in cell culture. As illustrated in Figure 3, although the inhibition of IL-6 and β-catenin increased the expression of Bax and decreased Bcl-2, this effect was not statistically significant. However, the combined treatment notably enhanced the expression levels of Bax while simultaneously reducing the expression levels of Bcl-2 (Figure 3).
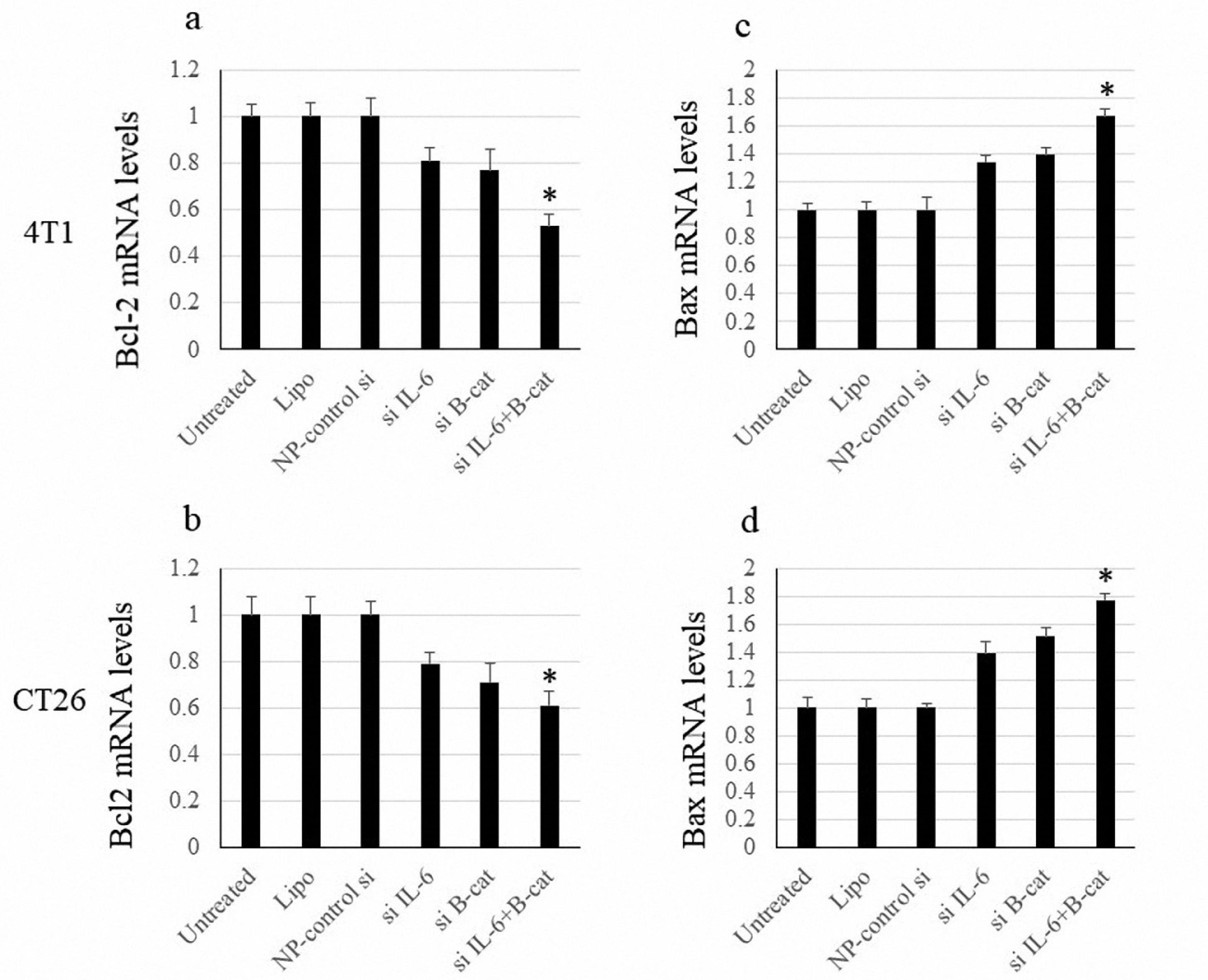
Figure 3.
Bax and Bcl-2 mRNA Levels. Note. SD: Standard deviation. Data show means ± SD of mRNA levels of Bcl-2 and Bax. The symbol * indicates P< 0.05
.
Bax and Bcl-2 mRNA Levels. Note. SD: Standard deviation. Data show means ± SD of mRNA levels of Bcl-2 and Bax. The symbol * indicates P< 0.05
The Effect of Combination Therapy on the Metastasis Gene Expression
The expression of MMP2 and MMP9 was evaluated as indicators for the metastasis process. Nonetheless, suppressing either IL-6 or β-catenin briefly reduced the expression of MMP2/9, where the combined blockade of IL-6 and β-catenin could significantly inhibit the MMP2/9 expression (Figure 4).
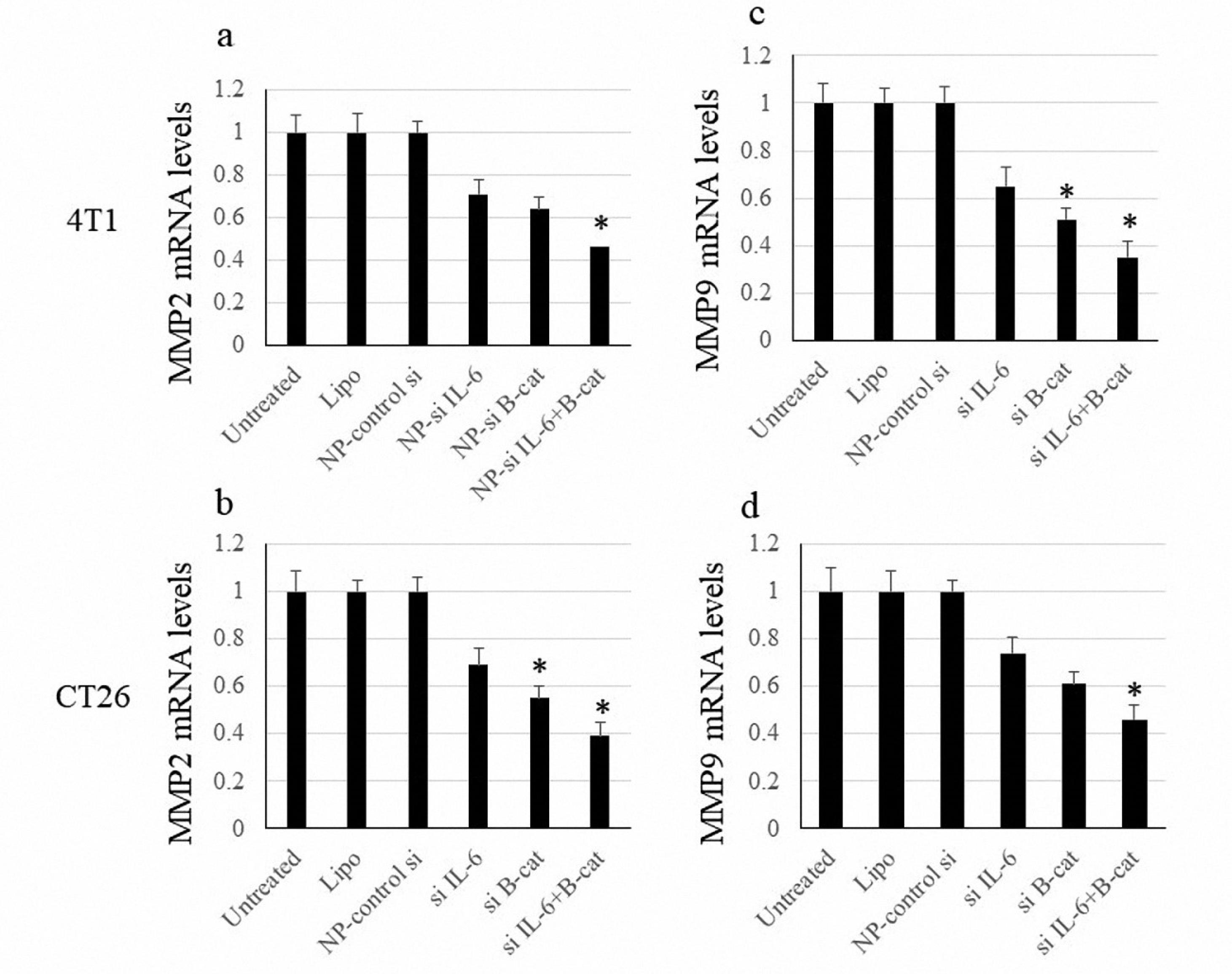
Figure 4.
MMP2 and MMP9 mRNA Levels. Note. SD: Standard deviation. The data are expressed as means ± SD of MMP2 and MMP9 expression. The symbol * represents P value < 0.05
.
MMP2 and MMP9 mRNA Levels. Note. SD: Standard deviation. The data are expressed as means ± SD of MMP2 and MMP9 expression. The symbol * represents P value < 0.05
Co-inhibition of Interleukin 6 or β-Catenin Decreases Angiogenesis-related Factors
The expression of vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) factors was studied to evaluate the anti-angiogenic impacts following siRNA transfection. Silencing either IL-6 or β-catenin decreased the expression of VEGF and FGF; however, this effect was not statistically significant. Meanwhile, the combined downregulation of IL-6 or β-catenin significantly suppressed the expression of VEGF and FGF (Figure 5).
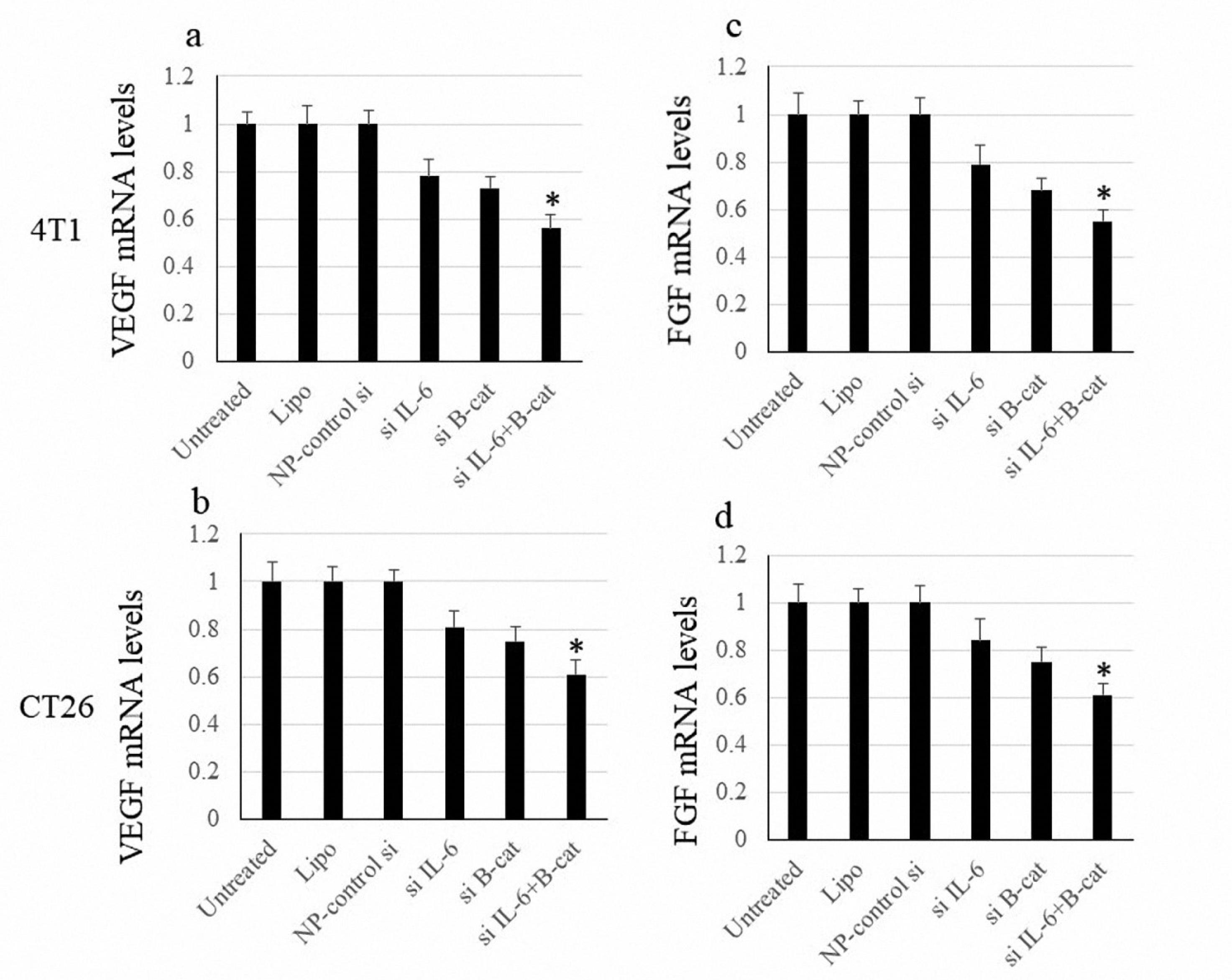
Figure 5.
FGF and VEGF mRNA Levels. Note. SD: Standard deviation; VEGF: Vascular endothelial growth factor; FGF: Fibroblast growth factor. The data are expressed as means ± SD of VEGF and FGF expression levels. The symbol * indicates P value < 0.05
.
FGF and VEGF mRNA Levels. Note. SD: Standard deviation; VEGF: Vascular endothelial growth factor; FGF: Fibroblast growth factor. The data are expressed as means ± SD of VEGF and FGF expression levels. The symbol * indicates P value < 0.05
Interleukin 6 and β-Catenin Co-blockade Inhibit Cell Proliferation
The anti-proliferative effect of combined β-catenin and IL-6 silencing was assessed using the 5-bromo-2-deoxyuridine assay. While IL-6 blockade significantly reduced cancer cell proliferation, downregulation of β-catenin briefly affected cells’ proliferation. Combined silencing of IL-6 and β-catenin exhibited the highest anti-proliferative potential among all therapeutic groups (Figure 6).
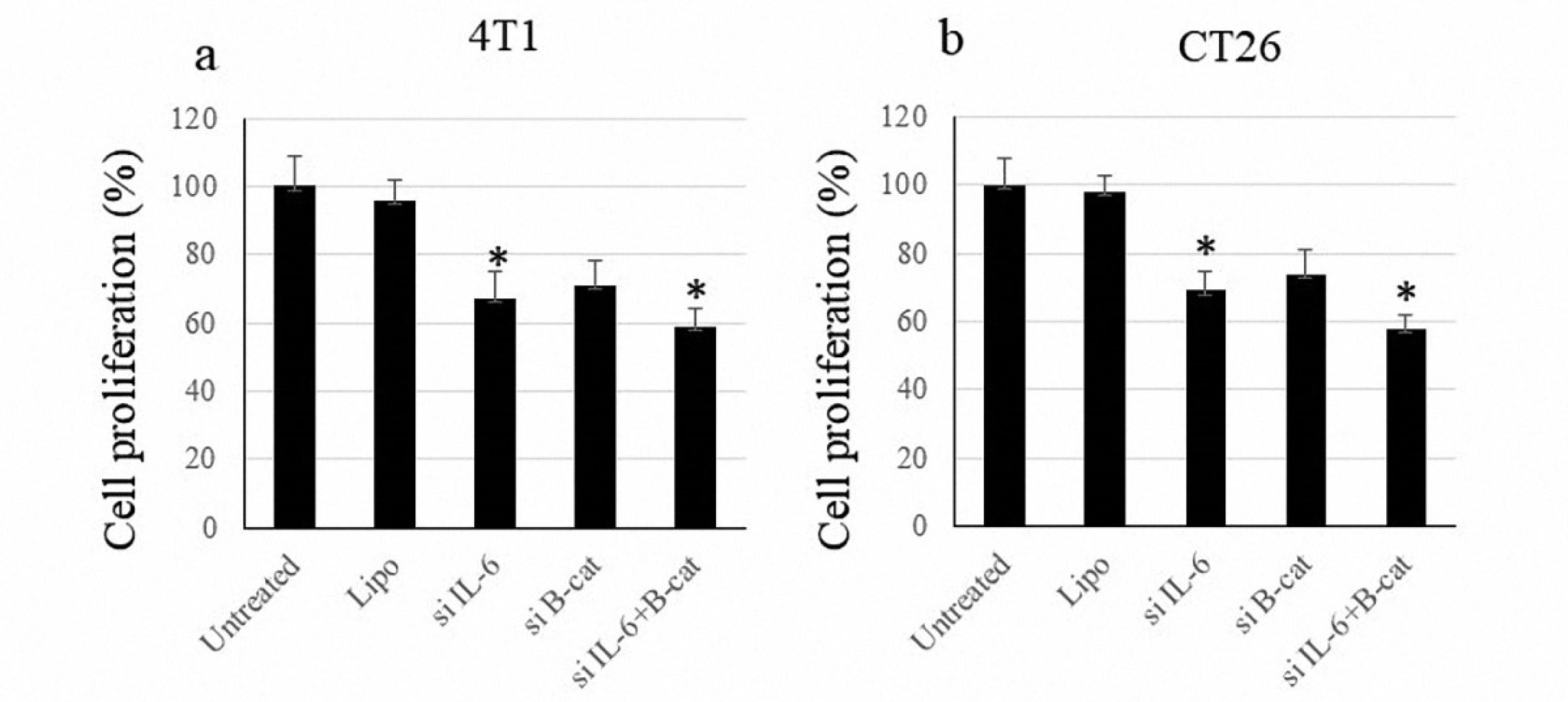
Figure 6.
Proliferation of Cancer Cells. Note. IL: Interleukin; SD: Standard deviation; siRNA: Small interfering RNA. The data are indicated as means ± SD of cancer cells’ proliferation following transfection with siRNA molecules against IL-6 and β-catenin. The symbol * represents P value < 0.05
.
Proliferation of Cancer Cells. Note. IL: Interleukin; SD: Standard deviation; siRNA: Small interfering RNA. The data are indicated as means ± SD of cancer cells’ proliferation following transfection with siRNA molecules against IL-6 and β-catenin. The symbol * represents P value < 0.05
Discussion
The upregulation of oncogenic elements within the TME poses a significant challenge to the success of diverse anti-cancer treatment modalities. Understanding the signaling cascades involved in tumor advancement is imperative for formulating efficacious therapeutic interventions.23 Currently, IL-6 is acknowledged as a significant autonomous pathway for biologically activating cytokine receptors. Nearly every cell in the body exhibits responsiveness to IL-6 signaling.10 IL can regulate the complex TME immune makeup and stimulate activation, inhibition, or differentiation based on specific cell types. IL-6 has a distinct and significant impact on the TME, acting as a key regulator by suppressing immune responses against tumors. The therapeutic targeting of IL-6-related cytokines and their receptors makes it possible to shift the equilibrium toward an antitumor TME. This approach presents a valid and promising alternative to hinder inflammation that promotes tumor growth.23 Recent studies have emphasized the significance of this approach in cancer development.
Over the past decade, therapies involving antibodies and small compounds targeting IL-6 signaling have been established as effective treatment options.10 However, a better understanding of how this cytokine family affects the TME in each stage is essential.24 Accordingly, Soler et al demonstrated that the suppression of these cytokines combined with immune checkpoint blockade results in enhanced immunotherapy outcomes.23 Furthermore, recent studies have underlined the promising potential of these cytokines as prognostic biomarkers for immune checkpoint blockade therapy effectiveness. This further reinforces their significant contribution to the overall efficacy of immunotherapies, highlighting their key role in achieving therapeutic success.25
The majority of clinical trials involving IL-6 blocking therapies typically involve patients with metastatic cancer. In fact, the process of neutralizing inflammatory cytokines leads to a consistent decrease in downstream cytokines present in the bloodstream. However, this approach only brings about temporary disease stabilization.26 Hoge et al found that focusing on IL-6 trans-signaling, specifically through the selective IL-6 inhibitor olamkicept,could provide therapeutic advantages by minimizing the common side effects associated with current anti-IL-6 treatments.27 Several researchers endeavored to improve anti-PD-1/PD-L1 and anti-CTLA-4 effectiveness agents by targeting IL-6 in melanoma and colon cancer, which has led to significantly lower cancer recurrence.28 IL-6 promotes tumor progression through various pathways and mechanisms, such as the induction of epithelial-mesenchymal transition (EMT) and macrophage polarization, whereas its blockade may also contribute to improved antitumor response.29,30 In addition, it was reported that the activation of the IL-6/JAK2/STAT3 signaling pathway can suppress bax/bcl2-associated caspase-dependent apoptosis, thereby facilitating the proliferation and metastasis of cancer cells.31 Another study indicated that the interaction between IL-6 and its receptor promotes the migration of chondrosarcoma by elevating the production of MMP-13.32
Identified decades ago, the pathway of Wnt/β-catenin signaling quickly captured considerable focus owing to its extensive role in regulating various aspects of tumorigenesis. This includes its influence on the disease’s initiation, development, progression, and diagnosis.33 The regulation of β-catenin’s activity involves a series of tightly controlled mechanisms, encompassing interactions between ligands and receptors, its stability, translocation into the nucleus, and transcriptional activity.34 Although the tissue-specific nature of the Wnt pathway provides several therapeutic advantages, it has turned it into a challenging target for developing safe and effective pharmacological agents.35
The intricacy of the Wnt signaling pathway provides numerous opportunities for therapeutic intervention. A broad range of interventions are being investigated to diminish the output of the Wnt/β-catenin signaling cascade, with several agents undergoing early clinical studies to evaluate their efficacy as potential treatments for cancer.36 Nevertheless, there are instances where these inhibitors, which aim to target early signaling events, may not be effective due to mutations that occur further downstream.16 The limited therapeutic advantages of Wnt/β-catenin signaling-associated targeted therapies in cancer can be attributed to their inadequate efficacy, specificity, and safety measures.33 For instance, through careful consideration, the therapeutic focus on β-catenin could potentially tackle challenges associated with stemness, plasticity, and resistance in cancerous cells, which are currently not effectively addressed by conventional modalities.37
Current evidence indicates that there is still a significant gap in the advancement of Wnt/β-catenin signaling targeted therapies for practical clinical application in cancer treatment.38 Despite the obstacles, primary studies have shown promising results in targeting Wnt/β-catenin signaling in vitro and in vivo. Additionally, some small molecule inhibitors and monoclonal antibodies designed to target Wnt/β-catenin signaling have demonstrated limited effectiveness due to the complexities involved in identifying specific structures and sites within the signaling components.33,39 It has been reported that the expression of matrix metalloproteinases mediated by β-catenin facilitates the process of EMT.40 Bi et al concluded that miR331-3p functions as a significant tumor suppressor by inhibiting MGAT1, which subsequently leads to the suppression of the Wnt/β-catenin signaling pathway and a reduction in the proliferation of osteosarcoma cells. This mechanism promotes increased apoptosis through the Bcl-2/Bax pathway and diminishes the migration and invasion capabilities of the cells via EMT.41 It is widely acknowledged that the abnormal activation of Wnt signaling serves a crucial function in the initiation and progression of diverse cancer kinds, including hepatocellular carcinoma, breast cancer, and colon carcinoma. The early findings connecting the activation of Wnt/β-catenin signaling to these cancers have established a strong foundation for this belief.42 For instance, significant endeavors have been undertaken to disrupt upstream Wnt signaling occurrences, encompassing Wnt lipidation, Wnt secretion, and interactions between ligands and receptors.43
Conclusion
Expanding upon these findings, it is postulated that targeting IL-6 may offer a potential avenue for reducing autoimmunity while simultaneously enhancing tumor immunity. Despite the extensive early research demonstrating the advantages of targeting Wnt/β-catenin signaling in cancer treatment, it remains crucial to explore the feasibility of developing therapies focusing on this pathway in the future. It is imperative for forthcoming studies to address the current challenges by minimizing adverse effects and enhancing the effectiveness and safety of IL-6 and β-catenin signaling targeted therapies for cancer.
Ethics statement
This study was approved by the Ethics Committee of Tabriz University of Medical Sciences (IR.TBZMED.VCR.REC.1401.149).
Conflict of interests declaration
The authors declare that they have no conflict of interests.
Acknowledgments
We would like to thank Tabriz University of Medical Sciences for the financial support of this study (Grant numbers: 69958 and 73516).
Data availability statement
The data related to the study are available upon a reasonable request from the corresponding author.
Author contributions
Conceptualization: Farhad Jadidi-Niaragh, Mohammad Hojjat-Farsangi, Maryam Farajzadeh.
Data curation: Maryam Farajzadeh, Mohammad-Salar Hosseini.
Funding acquisition: Farzaneh Eshaghi, Samira Mohebbi.
Investigation: Farzaneh Eshaghi, Samira Mohebbi.
Methodology: Maryam Farajzadeh, Mohammad-Salar Hosseini, Farzaneh Eshaghi, Samira Mohebbi.
Project administration: Farhad Jadidi-Niaragh.
Resources: Farhad Jadidi-Niaragh, Mohammad Hojjat-Farsangi.
Software: Mohammad-Salar Hosseini, Farzaneh Eshaghi, Samira Mohebbi.
Supervision: Farhad Jadidi-Niaragh.
Validation: Farhad Jadidi-Niaragh, Mohammad Hojjat-Farsangi.
Visualization: Farhad Jadidi-Niaragh, Mohammad Hojjat-Farsangi.
Writing–original draft: Maryam Farajzadeh.
Writing–review & editing: Maryam Farajzadeh, Mohammad-Salar Hosseini, Farhad Jadidi-Niaragh.
References
- Babar Q, Saeed A, Tabish TA, Sarwar M, Thorat ND. Targeting the tumor microenvironment: potential strategy for cancer therapeutics. Biochim Biophys Acta Mol Basis Dis 2023; 1869(6):166746. doi: 10.1016/j.bbadis.2023.166746 [Crossref] [ Google Scholar]
- Wang Q, Shao X, Zhang Y, Zhu M, Wang FX, Mu J. Role of tumor microenvironment in cancer progression and therapeutic strategy. Cancer Med 2023; 12(10):11149-65. doi: 10.1002/cam4.5698 [Crossref] [ Google Scholar]
- Prossomariti A, Piazzi G, Alquati C, Ricciardiello L. Are Wnt/β-catenin and PI3K/AKT/mTORC1 distinct pathways in colorectal cancer?. Cell Mol Gastroenterol Hepatol 2020; 10(3):491-506. doi: 10.1016/j.jcmgh.2020.04.007 [Crossref] [ Google Scholar]
- Khalaf K, Hana D, Chou JT, Singh C, Mackiewicz A, Kaczmarek M. Aspects of the tumor microenvironment involved in immune resistance and drug resistance. Front Immunol 2021; 12:656364. doi: 10.3389/fimmu.2021.656364 [Crossref] [ Google Scholar]
- Huynh J, Chand A, Gough D, Ernst M. Therapeutically exploiting STAT3 activity in cancer - using tissue repair as a road map. Nat Rev Cancer 2019; 19(2):82-96. doi: 10.1038/s41568-018-0090-8 [Crossref] [ Google Scholar]
- Bent EH, Millán-Barea LR, Zhuang I, Goulet DR, Fröse J, Hemann MT. Microenvironmental IL-6 inhibits anti-cancer immune responses generated by cytotoxic chemotherapy. Nat Commun 2021; 12(1):6218. doi: 10.1038/s41467-021-26407-4 [Crossref] [ Google Scholar]
- Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity 2019; 51(1):27-41. doi: 10.1016/j.immuni.2019.06.025 [Crossref] [ Google Scholar]
- Anderson R, Rapoport BL. Immune dysregulation in cancer patients undergoing immune checkpoint inhibitor treatment and potential predictive strategies for future clinical practice. Front Oncol 2018; 8:80. doi: 10.3389/fonc.2018.00080 [Crossref] [ Google Scholar]
- Korn T, Hiltensperger M. Role of IL-6 in the commitment of T cell subsets. Cytokine 2021; 146:155654. doi: 10.1016/j.cyto.2021.155654 [Crossref] [ Google Scholar]
- Rose-John S, Jenkins BJ, Garbers C, Moll JM, Scheller J. Targeting IL-6 trans-signalling: past, present and future prospects. Nat Rev Immunol 2023; 23(10):666-81. doi: 10.1038/s41577-023-00856-y [Crossref] [ Google Scholar]
- Garbers C, Heink S, Korn T, Rose-John S. Interleukin-6: designing specific therapeutics for a complex cytokine. Nat Rev Drug Discov 2018; 17(6):395-412. doi: 10.1038/nrd.2018.45 [Crossref] [ Google Scholar]
- RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2021; 397(10285):1637-45. doi: 10.1016/s0140-6736(21)00676-0 [Crossref] [ Google Scholar]
- Ma Q, Yu J, Zhang X, Wu X, Deng G. Wnt/β-catenin signaling pathway-a versatile player in apoptosis and autophagy. Biochimie 2023; 211:57-67. doi: 10.1016/j.biochi.2023.03.001 [Crossref] [ Google Scholar]
- Xue W, Yang L, Chen C, Ashrafizadeh M, Tian Y, Sun R. Wnt/β-catenin-driven EMT regulation in human cancers. Cell Mol Life Sci 2024; 81(1):79. doi: 10.1007/s00018-023-05099-7 [Crossref] [ Google Scholar]
- Sun L, Xing J, Zhou X, Song X, Gao S. Wnt/β-catenin signalling, epithelial-mesenchymal transition and crosslink signalling in colorectal cancer cells. Biomed Pharmacother 2024; 175:116685. doi: 10.1016/j.biopha.2024.116685 [Crossref] [ Google Scholar]
- Shang S, Hua F, Hu ZW. The regulation of β-catenin activity and function in cancer: therapeutic opportunities. Oncotarget 2017; 8(20):33972-89. doi: 10.18632/oncotarget.15687 [Crossref] [ Google Scholar]
- Dev A Jr, Vachher M, Prasad CP. β-catenin inhibitors in cancer therapeutics: intricacies and way forward. Bioengineered 2023; 14(1):2251696. doi: 10.1080/21655979.2023.2251696 [Crossref] [ Google Scholar]
- Zhang X, Wang L, Qu Y. Targeting the β-catenin signaling for cancer therapy. Pharmacol Res 2020; 160:104794. doi: 10.1016/j.phrs.2020.104794 [Crossref] [ Google Scholar]
- Bai G, Wang H, Cui N. mTOR pathway mediates endoplasmic reticulum stress-induced CD4( + ) T cell apoptosis in septic mice. Apoptosis 2022; 27(9-10):740-50. doi: 10.1007/s10495-022-01740-1 [Crossref] [ Google Scholar]
- Masjedi A, Ahmadi A, Atyabi F, Farhadi S, Irandoust M, Khazaei-Poul Y. Silencing of IL-6 and STAT3 by siRNA loaded hyaluronate-N,N,N-trimethyl chitosan nanoparticles potently reduces cancer cell progression. Int J Biol Macromol 2020; 149:487-500. doi: 10.1016/j.ijbiomac.2020.01.273 [Crossref] [ Google Scholar]
- Abolhasani S, Javadi S, Hassannia H, Ghalamfarsa G, Hojjat-Farsangi M, Jadidi-Niaragh F. IGF1R and HIF-1α gene silencing inhibits cancer cell growth. Int J Drug Res Clin 2023; 1(1):e17. doi: 10.34172/ijdrc.2023.e17 [Crossref] [ Google Scholar]
- Khodayari P, Khodakarami A, Hassannia H, Ghalamfarsa G, Hojjat-Farsangi M, Hashemi V. Silencing interleukin-6 and glycoprotein 130 suppresses growth and induces apoptosis in cancer cells. Int J Drug Res Clin 2023; 1(1):e8. doi: 10.34172/ijdrc.2023.e8 [Crossref] [ Google Scholar]
- Soler MF, Abaurrea A, Azcoaga P, Araujo AM, Caffarel MM. New perspectives in cancer immunotherapy: targeting IL-6 cytokine family. J Immunother Cancer 2023; 11(11):e007530. doi: 10.1136/jitc-2023-007530 [Crossref] [ Google Scholar]
- Garner H, de Visser KE. Immune crosstalk in cancer progression and metastatic spread: a complex conversation. Nat Rev Immunol 2020; 20(8):483-97. doi: 10.1038/s41577-019-0271-z [Crossref] [ Google Scholar]
- Rossi JF, Chiang HC, Lu ZY, Levon K, van Rhee F, Kanhai K. Optimisation of anti-interleukin-6 therapy: precision medicine through mathematical modelling. Front Immunol 2022; 13:919489. doi: 10.3389/fimmu.2022.919489 [Crossref] [ Google Scholar]
- Propper DJ, Balkwill FR. Harnessing cytokines and chemokines for cancer therapy. Nat Rev Clin Oncol 2022; 19(4):237-53. doi: 10.1038/s41571-021-00588-9 [Crossref] [ Google Scholar]
- Hoge J, Yan I, Jänner N, Schumacher V, Chalaris A, Steinmetz OM. IL-6 controls the innate immune response against Listeria monocytogenes via classical IL-6 signaling. J Immunol 2013; 190(2):703-11. doi: 10.4049/jimmunol.1201044 [Crossref] [ Google Scholar]
-
Hailemichael Y, Johnson DH, Abdel-Wahab N, Foo WC, Bentebibel SE, Daher M, et al. Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity. Cancer Cell 2022;40(5):509-23.e6. doi: 10.1016/j.ccell.2022.04.004.
- Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol 2015; 16(5):448-57. doi: 10.1038/ni.3153 [Crossref] [ Google Scholar]
- Wang J, Peng J, Chen Y, Nasser MI, Qin H. The role of stromal cells in epithelial-mesenchymal plasticity and its therapeutic potential. Discov Oncol 2024; 15(1):13. doi: 10.1007/s12672-024-00867-8 [Crossref] [ Google Scholar]
- Xie Q, Yang Z, Huang X, Zhang Z, Li J, Ju J. Ilamycin C induces apoptosis and inhibits migration and invasion in triple-negative breast cancer by suppressing IL-6/STAT3 pathway. J Hematol Oncol 2019; 12(1):60. doi: 10.1186/s13045-019-0744-3 [Crossref] [ Google Scholar]
- Tang CH, Chen CF, Chen WM, Fong YC. IL-6 increases MMP-13 expression and motility in human chondrosarcoma cells. J Biol Chem 2011; 286(13):11056-66. doi: 10.1074/jbc.M110.204081 [Crossref] [ Google Scholar]
- Yu F, Yu C, Li F, Zuo Y, Wang Y, Yao L. Wnt/β-catenin signaling in cancers and targeted therapies. Signal Transduct Target Ther 2021; 6(1):307. doi: 10.1038/s41392-021-00701-5 [Crossref] [ Google Scholar]
- Song P, Gao Z, Bao Y, Chen L, Huang Y, Liu Y. Wnt/β-catenin signaling pathway in carcinogenesis and cancer therapy. J Hematol Oncol 2024; 17(1):46. doi: 10.1186/s13045-024-01563-4 [Crossref] [ Google Scholar]
- Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang X. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther 2022; 7(1):3. doi: 10.1038/s41392-021-00762-6 [Crossref] [ Google Scholar]
- Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol 2009; 5(2):100-7. doi: 10.1038/nchembio.137 [Crossref] [ Google Scholar]
- Lei ZN, Tian Q, Teng QX, Wurpel JND, Zeng L, Pan Y. Understanding and targeting resistance mechanisms in cancer. MedComm (2020) 2023; 4(3):e265. doi: 10.1002/mco2.265 [Crossref] [ Google Scholar]
- Choudhary S, Singh MK, Kashyap S, Seth R, Singh L. Wnt/β-catenin signaling pathway in pediatric tumors: implications for diagnosis and treatment. Children (Basel) 2024; 11(6):700. doi: 10.3390/children11060700 [Crossref] [ Google Scholar]
- Yan M, Li G, An J. Discovery of small molecule inhibitors of the Wnt/β-catenin signaling pathway by targeting β-catenin/Tcf4 interactions. Exp Biol Med (Maywood) 2017; 242(11):1185-97. doi: 10.1177/1535370217708198 [Crossref] [ Google Scholar]
- Orlichenko LS, Radisky DC. Matrix metalloproteinases stimulate epithelial-mesenchymal transition during tumor development. Clin Exp Metastasis 2008; 25(6):593-600. doi: 10.1007/s10585-008-9143-9 [Crossref] [ Google Scholar]
- Bi W, Yang M, Xing P, Huang T. MicroRNA miR-331-3p suppresses osteosarcoma progression via the Bcl-2/Bax and Wnt/β-Catenin signaling pathways and the epithelial-mesenchymal transition by targeting N-acetylglucosaminyltransferase I (MGAT1). Bioengineered 2022; 13(6):14159-74. doi: 10.1080/21655979.2022.2083855 [Crossref] [ Google Scholar]
- Luke JJ, Bao R, Sweis RF, Spranger S, Gajewski TF. WNT/β-catenin pathway activation correlates with immune exclusion across human cancers. Clin Cancer Res 2019; 25(10):3074-83. doi: 10.1158/1078-0432.ccr-18-1942 [Crossref] [ Google Scholar]
- Werner J, Boonekamp KE, Zhan T, Boutros M. The roles of secreted Wnt ligands in cancer. Int J Mol Sci 2023; 24(6):5349. doi: 10.3390/ijms24065349 [Crossref] [ Google Scholar]