Int J Drug Res Clin. 2:e21.
doi: 10.34172/ijdrc.2024.e21
Original Article
Effect of Saffron and Melissa officinalis L. Supplementation on Glycemic Parameters and Lipid Profile in Patients with Type 2 Diabetes Mellitus: A Double-Blind Clinical Trial
Behnam Sadighi 1  , Reza Yarani 2, 3, Somaiyeh Taheri-Targhi 4, Sarvin Sanaie 5, Seyed Kazem Shakouri 6, Majid Mobasseri 7, Zahra Yousefi 4, *
, Reza Yarani 2, 3, Somaiyeh Taheri-Targhi 4, Sarvin Sanaie 5, Seyed Kazem Shakouri 6, Majid Mobasseri 7, Zahra Yousefi 4, *  , Mostafa Araj-Khodaei 8, *
, Mostafa Araj-Khodaei 8, * 
Author information:
1Medicinal Plants Research Center, Maragheh University of Medical Sciences, Maragheh, Iran.
2Translational Type 1 Diabetes Research, Department of Clinical Research, Steno Diabetes Center Copenhagen, Gentofte, Denmark
3Deakin University, IMPACT – The Institute for Mental and Physical Health and Clinical Translation, Food & Mood Centre, School of Medicine, Barwon Health, Geelong, Victoria, Australia
4Research Center of Psychiatry and Behavioral Sciences, Aging Research Institute, Tabriz University of Medical Sciences, Tabriz, Iran
5Neurosciences Research Center, Aging Research Institute, Tabriz University of Medical Sciences, Tabriz, Iran
6Physical Medicine and Rehabilitation Research Center, Aging Research Institute, Tabriz University of Medical Sciences, Tabriz, Iran
7Department of Internal Medicine, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
8Research Center for Integrative Medicine in Aging, Aging Research Institute, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Background:
Diabetes is one of the most common diseases in modern societies. The prevalence of type 2 diabetes mellitus (T2DM) is rising and is expected to reach 440 million people by 2030. Although effective steps have been taken in diabetes treatment using common chemical drugs, these drugs are often followed by numerous side effects, necessitating the search for new medications with natural origin, especially those derived from traditional and complementary medicine.
Methods:
In this double-blind clinical trial, 90 type 2 diabetic patients were randomly divided into three groups: saffron (30 patients), Melissa officinalis (29 patients), and placebo (28 patients). After obtaining informed written consent from each patient, blood samples were collected from all participants, and the corresponding drug was delivered to each patient. After the participants took the medications for three months, blood samples were taken again to analyze the biochemical parameters. Data were analyzed using SPSS version 24, employing both descriptive and inferential statistics.
Results:
At the outset of the investigation, no significant differences existed among the three groups regarding the pertinent variables. The results showed that saffron supplement and M. officinalis significantly decreased fasting blood sugar (FBS) (P<0.001), hemoglobin A1C (HbA1c) (P<0.05), triglyceride (P<0.05), and total cholesterol (P<0.001) compared to pre-intervention stage. No significant effects were observed for other parameters.
Conclusion:
This study demonstrated that lemongrass and saffron extracts significantly affected the control of FBS, HbA1c, triglycerides, and blood cholesterol levels in type 2 diabetes patients, suggesting their administration alongside other treatments.
Keywords: Type 2 diabetes, Herbal medicine, Saffron, Melissa officinalis, Nutrition
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
The research protocol was approved and supported by the Tabriz University of Medical Sciences (Grant number 64796).
Please cite this article as follows: Sadighi B, Yarani R, Taheri-Targhi S, Sanaie S, Shakouri SK, Mobasseri M, et al. Effect of saffron and Melissa officinalis L. Supplementation on glycemic parameters and lipid profile in patients with type 2 diabetes mellitus: a double-blind clinical trial. Int J Drug Res Clin. 2024; 2: e21. doi: 10.34172/ijdrc.2024.e21
Introduction
Diabetes is a prevalent metabolic disorder that can develop when the pancreas does not generate enough insulin or when the body cannot properly utilize the generated insulin, leading to an increase in blood sugar and disturbances in carbohydrate, fat, and protein metabolism.1 Carbohydrates are divided by humans into simpler forms of sugars. Pancreatic beta cells release insulin.2 Lack of insulin or insensitivity of its receptors may cause continuous or frequent hyperglycemia, which eventually leads to diabetes. Symptoms of diabetes mellitus include polyuria, polydipsia, weight loss, and sometimes polyphagia and blurred vision. Susceptibility to some infections may also be a symptom of chronic hyperglycemia.3 Type 2 diabetes includes 90 to 95% of all diabetes cases and occurs in people who are resistant to insulin and usually have a relative insulin deficiency in the beginning. These people usually do not need insulin treatment.4 Premature mortality, as well as heart, kidney, nervous system diseases, and blindness in diabetic people, is twice as high as in non-diabetic people.5 The World Health Organization (WHO) reports that about 2% of deaths in our country are caused by diabetes. A 2017 report published by the WHO indicated a substantial rise among 18-year-olds diagnosed with diabetes from 4.7% in 1980 to 8.5% in 2014 globally.6 Although insulin and oral antidiabetic drugs are crucial for the treatment of diabetes, complications from these medications have remained stable. Studies reveal that chemical drugs, which may have side effects, are inadequate in controlling glucose levels in some patients (1,5). In addition, comprehensive and completely effective treatments for those suffering from multiple metabolic disorders are lacking.7 Consequently, due to the high prevalence of diabetes and its complications, physicians are looking for alternative or complementary medicines, especially medicinal plants, which have historically played a significant role in treating various health issues.8 Saffron (Crocus sativus L), a member of the Iridaceae family, is predominantly cultivated in Iran, India, Greece, Italy, Spain, and France.9 Its therapeutic value is attributed to the presence of four main metabolites: crocin, crostin, picrocrocin, and safranal. Flavonoids and carotenoids have also been found in saffron.10 In addition to antioxidant properties, these components have different properties such as reducing serum lipids and protecting the heart, nerves, and liver, along with anti-cancer, insulin-sensitizing, and anti-inflammatory effects.11 In vitro and in vivo investigations have revealed that saffron and its compounds can influence hyperglycemia and exhibit antidiabetic properties.12 Melissa officinalis L. belonging to the mint family, is a perennial plant that can grow up to 100 cm. This species originates from southern Europe, Asia, and parts of North and South America. M. officinalis is found throughout the Mediterranean region, extending to the Turkish coastline and northern Iran.13 This plant is rich in flavonoids known for their ability to lower fat and blood sugar levels.14 Numerous studies have confirmed the effects of flavonoids on blood sugar reduction.15,16 Research has shown that lemon scent contains substantial amounts of phenolic and flavonoid compounds, including rosmarinic acid and caffeic acid,14 which have antioxidant, antidiabetic, and blood pressure-lowering effects.17 Several animal and laboratory studies have demonstrated that M. officinalis exhibits hypoglycemic, hypolipidemic,13 antiglycation activity,18 and pancreatic amylase inhibitory activity. Research has indicated that lemongrass essential oil can reduce blood sugar due to its antioxidant properties. Since oxidative stress and reactive oxygen species can cause diabetes and related complications, the proper consumption of natural antioxidants such as lemon essential oil may help prevent or improve diabetes symptoms or its complications.13 Therefore, the main purpose of the study was to investigate the effects of M. officinalis and saffron on controlling blood sugar factors and lipid profiles in patients with type 2 diabetes.
Hypothesis
-
Fasting blood sugar (FBS) levels differ between study groups before and after the intervention.
-
HbA1c levels differ between study groups before and after the intervention.
-
Lipid profile levels differ between study groups before and after the intervention.
Methods and Materials
Study Design and Participants
A 3-month double-blind, randomized clinical trial was conducted at Imam Reza Hospital in Tabriz, Iran, from July 2020 to July 2021. The research protocol was approved by Tabriz University of Medical Sciences (Grant No. 64796). Patients were fully informed about the trial’s content and procedures and provided written consent. The study adhered to the revised Helsinki Declaration and was registered in the Iranian Clinical Trials Registry (identifier: IRCT20140617018126N4). The Ethics Committee of Tabriz University of Medical Sciences endorsed the study protocol after a thorough evaluation (IR.TBZMED.REC.1399.160).
Inclusion Criteria
-
Individuals with type 2 diabetes (FBS levels > 126 mg/dL).
-
Taking oral hypoglycemic drugs.
-
Aged 40-70 years.
-
Body mass index (BMI) of 18/5-30 kg/m2.
-
Willingness to participate in the research.
Exclusion Criteria
-
Diagnosed with type 1 diabetes or other unique forms of diabetes.
-
HbA1c higher than 9%
-
Insulin treatment within the last three months
-
Diagnosed with significant gastrointestinal disorders (e.g., stomach ulcers and gastrointestinal hemorrhage).
-
History of diabetic ketoacidosis, non-ketotic hyperosmolar coma, significant infections, or surgeries in the past month.
-
History of mental illness.
-
Misuse or abuse of alcohol, drugs, or psychedelics.
-
Pregnancy, lactation, or planning to become pregnant.
-
Disorders affecting the cardiovascular system, kidney function, liver health, thyroid and parathyroid glands, or cancer.
-
Chronic complications of diabetes (neuropathy, retinopathy, as per patient records), diabetic nephropathy, hypothyroidism, and hyperthyroidism.
-
Allergy to saffron or M. officinalis.
Sample Size
The sample size was determined using G-POWER software, based on previous research.19-21 Considering the FBS parameters (power = 95%, α = 0.05, SD = 5.28), the sample size was calculated to be 27 patients for each group. Given a potential dropout rate of 15%, 90 participants were randomly allocated into three groups (n = 30 each), with 87 participants remaining until the end of the study.
Randomization and Random Allocation
Participants were randomly assigned to one of the three treatment groups using a randomized block procedure (Random Allocation Software). Concealment procedures, including blocking and allocation were executed by a researcher not involved in a study. The allocation ratio was 1:1:1, assigning participants to three groups: receiving saffron, M. officinalis, and placebo. Neither researchers nor patients were aware of the medication each person received.
Measurements
Demographic details such as age, gender, education level, past medication history, and consumption of dietary supplements, along with the details of their dosages and duration were gathered via a self-report questionnaire. Additional data on past adherence to particular diets, current health conditions (e.g., diabetes and other disorders), and previous diabetes history were also gathered. Then, fasting blood samples were taken, and the weight, height, BMI, waist measurement, wrist size, urine samples, and blood pressure were recorded. The medication intake questionnaire was employed to gather information about the medications used by participants. A checklist of potential drug side effects was distributed to all patients involved in the study. Physicians were responsible for completing this checklist, and patients could contact their physician by phone for consultations.
Treatment Medications
The physical characteristics of saffron, M. officinalis, and placebo capsules were indistinguishable. The capsules, enclosed in white boxes, were to be taken by subjects alongside their food intake. Patients in the saffron group received 250 mg of saffron hydroalcoholic extract capsules every 12 hours for three months. The M. officinalis group received M. officinalis hydroalcoholic extract capsules every 12 hours for three months, while the placebo group also received two capsules of 250 mg placebo every 12 hours for three months. Patients were instructed to maintain their regular diets and physical activity routines throughout the intervention and were monitored via weekly phone calls to report any side effects.
The saffron extract capsules were obtained from Sina No Andish Company, and the raw materials of M. officinalis were obtained from rural areas in East Azerbaijan, where they were crushed, subsequently soaked in 70% alcohol for 48 hours, and filtered. The solvent was extracted using a rotary, and the extract was dried. Further, cornstarch flour was used to prepare the placebo.
Statistical Analysis
The Kolmogorov-Smirnov test was utilized to investigate the normality of the data. One-way ANOVA and, if necessary, the Kruskal-Wallis test were employed to compare the groups at baseline and the research outcomes at the end of the trial. The chi-square test was utilized to analyze qualitative data differences between groups. The results were expressed as mean ± standard deviation, with a significance level of P < 0.05. Data were analyzed using the SPSS software version 23.
Results
A total of 97 individuals enrolled in this study. However, three participants from the intervention group discontinued their participation due to personal reasons. The final number of participants in the intervention groups was 30 in the saffron group, 29 in the M. officinalis group, and 28 in the placebo group (Figure 1).
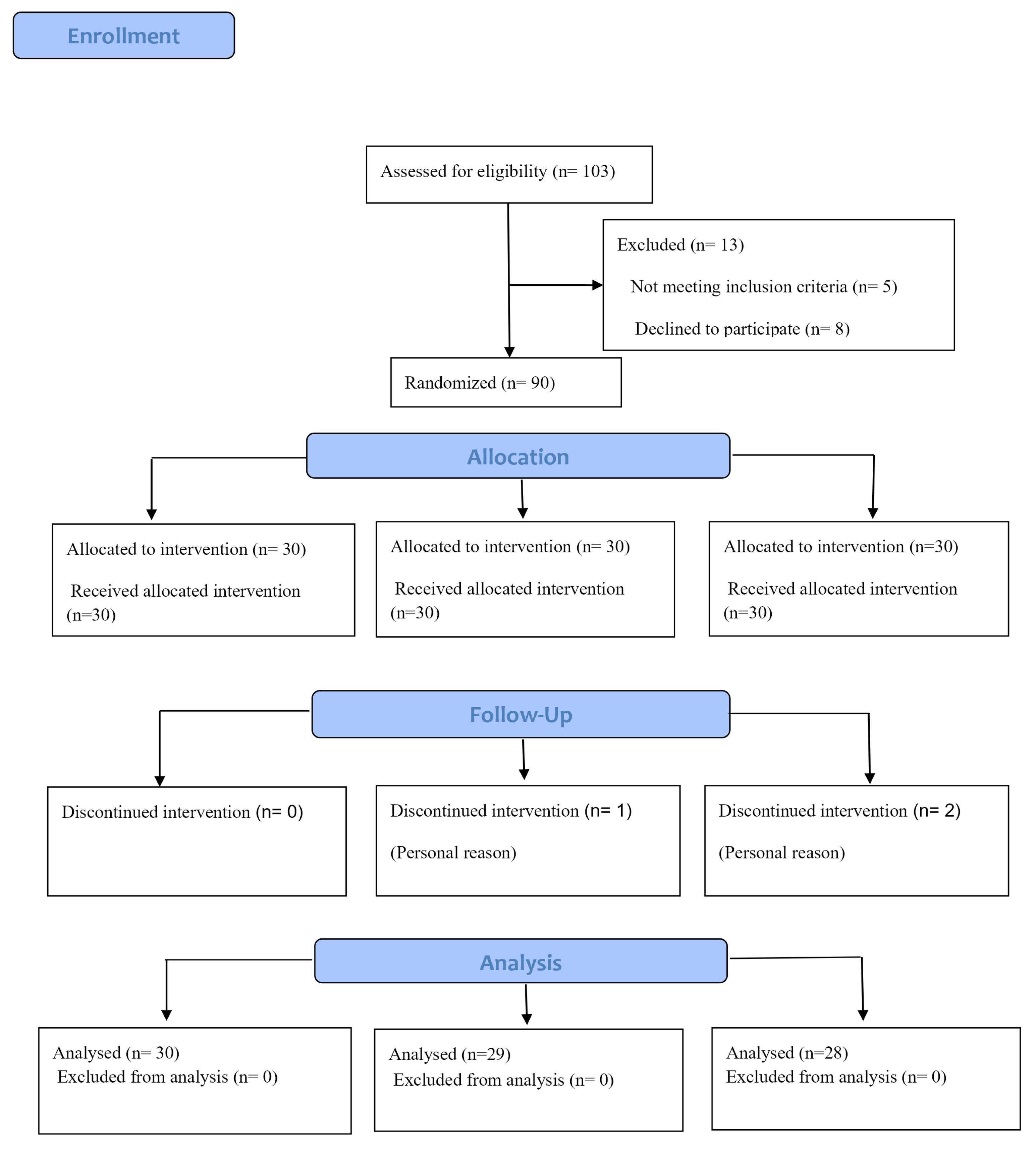
Figure 1.
Consort Flow Diagram of the Study
.
Consort Flow Diagram of the Study
The results demonstrated that baseline characteristics do not differ significantly between the study groups, except for the presence of other diseases (P = 0.016), alanine aminotransferase (ALT) (P = 0.008), alkaline phosphatase (P = 0.01), and Urea (0.015), as illustrated in Table 1.
Table 1.
Baseline Characteristics of Participants in the Survey
|
Variable
|
Group
|
P
Value
|
|
Saffron (n=30)
|
Melissa officinalis (n=29)
|
Placebo (n=28)
|
| Age |
|
60.6 ± 6.9 |
59.1 ± 9.22 |
60.4 ± 6.3 |
0.52 |
| Gender |
Male |
19 |
17 |
18 |
0.225 |
| Female |
11 |
12 |
10 |
| Family History of Diabetes |
Yes |
23 |
19 |
17 |
0.408 |
| No |
7 |
10 |
11 |
| Other Diseases |
Hyperlipidemia |
0 |
0 |
1 |
0.016 |
| Hypertension and hyperlipidemia |
4 |
0 |
4 |
| Acute coronary syndrome |
0 |
1 |
0 |
| Other |
8 |
13 |
8 |
| None |
8 |
6 |
13 |
| BMI |
|
29.92 ± 4.3 |
29.56 ± 3.91 |
28.17 ± 2.5 |
0.173 |
| FBS |
|
163.06 ± 44.3 |
160.2 ± 36.8 |
160.8 ± 61.9 |
0.973 |
| HbA1c |
|
7.04 ± 1.2 |
7.1 ± 0.77 |
7.4 ± 1.15 |
0.615 |
| Insulin |
|
9.78 ± 3.9 |
10. 97 ± 4.1 |
11.79 ± 5.16 |
0.078 |
| Insulin resistance |
|
4.28 ± 1.17 |
4.32 ± 2.41 |
4.5 ± 2.13 |
0.054 |
| Triglycerides |
|
169.7 ± 93.8 |
181.7 ± 49.9 |
171.2 ± 30.8 |
0.147 |
| Cholesterol |
|
165.3 ± 48.9 |
159.5 ± 34.6 |
161.2 ± 86.9 |
0.744 |
| HDL |
|
38.42 ± 5.5 |
38.37 ± 6.25 |
39.5 ± 3.3 |
0.854 |
| LDL |
|
94.6 ± 38.5 |
85.13 ± 33.44 |
100.5 ± 18.2 |
0.051 |
| Urea |
|
32.1 ± 6.6 |
27.4 ± 3.1 |
33.0 ± 9.5 |
0.015 |
| BUN |
|
14.96 ± 3.25 |
15.8 ± 2.5 |
16.8 ± 5.4 |
0.082 |
| Creatinine |
|
0.76 ± 0.73 |
0.81 ± 0.21 |
0.8 ± 0.12 |
0.265 |
| AST |
|
32.2 ± 8.3 |
20.3 ± 6.04 |
18.4 ± 8.0 |
0.01 |
| ALT |
|
27.7 ± 11.5 |
30.3 ± 12.8 |
20.5 ± 7.7 |
0.008 |
| ALP |
|
167.2 ± 41.6 |
178.1 ± 25.9 |
177.8 ± 2 |
0.32 |
Note. BMI: Body mass index; FBS: Fasting blood sugar; HbA1c: Hemoglobin A1C; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; BUN: Blood urea nitrogen; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; ALP: Alkaline phosphatase.
Following a three-month treatment period, a significant difference was observed in mean FBS levels among the three research groups (P < 0.001), demonstrating that saffron and M. officinalis were notably effective in alleviating FBS compared to the placebo (P < 0.05). Additionally, saffron and M. officinalis exhibited statistically significant reductions in HbA1c compared to the placebo (P < 0.05). Notably, the M. officinalis group showed statistically significant changes in insulin resistance levels compared to baseline (P = 0.025). Further, the reduction in the triglyceride levels for the saffron and M. officinalis groups was 13.1 ± 31.9 and 12.3 ± 73.9 units, respectively, representing a significant difference from the pre-intervention stage. Moreover, saffron and M. officinalis could decrease average cholesterol levels by 16.0 ± 38.3 and 19.4 ± 33.3 units, respectively, after the intervention. The results revealed that changes in high-density lipoprotein (HDL) and urea levels among the three groups were statistically significant (P = 0.004; P < 0.001), with the M. officinalis group exhibiting significantly greater changes than the placebo group (P < 0.01). Following the intervention, a considerable difference was detected in the blood creatinine levels of the three study groups (P = 0.025), and the intervention led to a significant reduction in the average creatinine levels for both the saffron and M. officinalis groups (P < 0.05). After the intervention, considerable distinctions were observed in ALT levels among the three groups (P < 0.05). Hence, the blood ALT levels in the saffron and M. officinalis groups following the intervention were significantly lower than their respective levels before the intervention. The alkaline phosphatase levels were similar among the three groups before the intervention (P < 0.05), but following the intervention, a significant difference was observed (P < 0.001). No significant differences were observed in insulin, low-density lipoprotein (LDL), and alkaline phosphatase levels among the three study groups (P > 0.05), as depicted in Table 2.
Table 2.
Changes in the Understudy Groups Before and After Intervention
|
Variable
|
Stage
|
Group
|
P
Value for Changes
|
|
Saffron (n=30)
|
Melissa officinalis
(n=29)
|
Placebo (n=28)
|
| FBS |
Before |
163.06 ± 44.3 |
160.2 ± 36.8 |
160.8 ± 61.9 |
0.973 |
| After |
110.3 ± 21.7 |
134.9 ± 20.6 |
158.8 ± 56.9 |
˂0.001 |
| Changes |
52.9 ± 43.9 |
25.37 ± 29.2 |
2.00 ± 8.95 |
< 0.001 |
| HbA1c |
Before |
7.04 ± 1.2 |
7.1 ± 0.77 |
7.4 ± 1.15 |
0.615 |
| After |
6.5 ± 0415 |
6.66 ± 0.41 |
7.3 ± 0.89 |
< 0.001 |
| Changes |
0.54 ± 1.12 |
0.43 ± 0.83 |
0.1 ± 0.71 |
0.021 |
| Insulin |
Before |
9.78 ± 3.9 |
10.97 ± 4.1 |
11.79 ± 5.16 |
0.078 |
| After |
8.52 ± 2.29 |
9.72 ± 4.86 |
10.85 ± 2.66 |
0.003 |
| Changes |
1.26 ± 3.99 |
1.25 ± 3.97 |
0.93 ± 4.6 |
0.92 |
| Insulin resistance |
Before |
4.28 ± 1.17 |
4.32 ± 2.41 |
4.5 ± 2.13 |
0.054 |
| After |
4.17 ± 26.08 |
3.98 ± 0.35 |
4.41 ± 1.52 |
0.182 |
| Changes |
0.11 ± 0.6 |
0.34 ± 0.73 |
0.09 ± 1.75 |
0.075 |
| Triglyceride |
Before |
169.7 ± 93.8 |
181.7 ± 49.9 |
171.2 ± 30.8 |
0.147 |
| After |
156.6 ± 86.9 |
169.4 ± 86.2 |
169.1 ± 33.1 |
0.019 |
| Changes |
13.1 ± 31.9 |
12.3 ± 73.9 |
2.1 ± 12.5 |
0.1 |
| Cholesterol |
Before |
165.3 ± 48.9 |
159.5 ± 34.6 |
161.2 ± 86.9 |
0.744 |
| After |
149.3 ± 31.6 |
140.1 ± 34.7 |
153.5 ± 21.2 |
0.104 |
| Changes |
16.0 ± 38.3 |
19.4 ± 33.3 |
7.7 ± 17.1 |
0.037 |
| HDL |
Before |
38. 42 ± 5.5 |
38.37 ± 6.25 |
39.5 ± 3.3 |
0.854 |
| After |
39.7 ± 6.6 |
42.44 ± 4.5 |
40.3 ± 2.5 |
< 0.001 |
| Changes |
1.3 ± 8.4 |
3.9 ± 5.2 |
0.8 ± 5.6 |
0.004 |
| LDL |
Before |
94.6 ± 38.5 |
85.13 ± 33.44 |
100.5 ± 18.2 |
0.051 |
| After |
82.2 ± 27.8 |
72.04 ± 30.9 |
89/9 ± 14.8 |
0.437 |
| Changes |
12.4 ± 38.4 |
13.09 ± 33.0 |
10.6 ± 13.2 |
0.089 |
| Urea |
Before |
32.1 ± 6.6 |
27.4 ± 3.1 |
33.0 ± 9.5 |
0.015 |
| After |
31.4 ± 5.5 |
36.27 ± 5.97 |
32.7 ± 8.3 |
0.017 |
| Changes |
-0.73 ± 6.96 |
8.8 ± 4.8 |
-0.32 ± 6.58 |
< 0.001 |
| BUN |
Before |
14.96 ± 3.25 |
15.8 ± 2.5 |
16.8 ± 5.4 |
0.082 |
| After |
15.13 ± 2.6 |
16.3 ± 2.9 |
16.92 ± 4.1 |
0.056 |
| Changes |
0.17 ± 3.42 |
0.5 ± 4.07 |
0.12 ± 3.64 |
0.127 |
| Creatinine |
Before |
0.76 ± 0.73 |
0.81 ± 0.21 |
0.8 ± 0.12 |
0.265 |
| After |
0.68 ± 0.08 |
0.74 ± 0.11 |
0.75 ± 0.1 |
0.025 |
| Changes |
0.08 ± 0.06 |
0.07 ± 0.18 |
0.05 ± 0.07 |
0.475 |
| AST |
Before |
32.2 ± 8.3 |
20.3 ± 6.04 |
18.4 ± 8.0 |
0.01 |
| After |
19.6 ± 4.9 |
19.58 ± 4.2 |
20.0 ± 6.0 |
0.971 |
| Changes |
3.6 ± 8.52 |
0.75 ± 8.33 |
1.67 ± 4.06 |
0.056 |
| ALT |
Before |
27.7 ± 11.5 |
30.3 ± 12.8 |
20.5 ± 7.7 |
0.008 |
| After |
20.1 ± 7.7 |
25/75 ± 6/8 |
24.3 ± 9.0 |
0.005 |
| Changes |
7.6 ± 11.3 |
4.5 ± 10.46 |
3/82 ± 11.1 |
< 0.001 |
| ALP |
Before |
167.2 ± 41.6 |
178.1 ± 25.9 |
177.8 ± 2 |
0.32 |
| After |
173.7 ± 45.4 |
176.4 ± 30.5 |
182.3 ± 33.5 |
< 0.001 |
| Changes |
6.5 ± 21.3 |
1.7 ± 34.1 |
4.5 ± 31.7 |
< 0. 095 |
Note. FBS: Fasting blood sugar; HbA1c: Hemoglobin A1C; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; BUN: Blood urea nitrogen; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; ALP: Alkaline phosphatase.
Discussion
Considering the expanding trend towards traditional and complementary medicine, this study was designed to explore the effects of saffron and M. officinalis on glycemic parameters and lipid profiles in patients with type 2 diabetes. The statistical analysis demonstrated no significant difference in the mean FBS levels before intervention across the three groups, indicating that the groups were homogeneous. Following the intervention, the saffron and M. officinalis groups showed significantly lower average FBS levels compared to the placebo group. Additionally, the results indicated that the average FBS in the saffron group was significantly lower than that in the M. officinalis group, indicating a stronger effect of saffron on regulating FBS. These results suggest that saffron and M. officinalis use could decrease FBS in diabetic patients, which is consistent with the results of other studies. It can be inferred that saffron, through its anti-inflammatory mechanisms, may decrease the prevalence of inflammatory factors and consequently manage related factors such as blood sugar.22 Furthermore, the results demonstrated that oral intake of M. officinalis extract results in a decrease in fasting glucose levels for individuals with type 2 diabetes mellitus (T2DM), consistent with past research.23 Studies indicated that M. officinalis may contribute to lowering blood sugar through increased production of hepatic glucokinase and GLUT4 transporter, diminished expression of glucose-6-phosphatase (G6Pase), and a decrease in the expression of phosphoenolpyruvate carboxykinase.24 Regarding the impact of saffron and M. officinalis on HbA1c levels in individuals with type 2 diabetes, the research results indicated a notable reduction in HbA1c levels for both M. officinalis and saffron, both within and between the study groups. Findings from a study conducted by Asadi et al in 2018 revealed that saffron intake leads to a substantial decrease in HbA1c levels.25 Similarly, the results of another study demonstrated that saffron has a significant effect on both FBS and HbA1c levels.26 However, a study conducted by Barari et al in 2018 revealed no significant reduction in HbA1c levels due to saffron intake and aerobic exercise, which is inconsistent with our study results 27. The discrepancies in results may be due to the variations in study duration and saffron dosage. Regarding the effect of M. officinalis on HbA1c, findings indicated that consuming M. officinalis oral capsules decreases the HbA1c levels in patients with type 2 diabetes, aligning with results from other studies. The inhibitory effect of M. officinalis on pancreatic alpha-amylase and alpha-glucosidase may explain the reduction in HbA1c and post-meal blood sugar levels in our study. According to our findings, saffron consumption led to a significant reduction in average triglyceride and total cholesterol levels, while exerting no significant effect on LDL and HDL levels. Research conducted by Tajaddini et al indicated that saffron intake effectively reduces blood triglyceride levels in diabetic patients, as observed in our study.28 However, another study revealed that saffron consumption for 12 weeks does not yield significant changes in lipid profiles. Moreover, Asbaghi et al reported no significant change in lipid levels after administering saffron hydroalcoholic extract.29 Due to these inconclusive findings, further research is required to provide a clearer understanding of the effect of saffron on lipid profile. In addition, the results revealed that the triglyceride and cholesterol concentrations in the M. officinalis group decreased significantly after the intervention. Although a significant increase in HDL concentration was observed, it had no impact on LDL levels. The anti-hyperlipidemic effects of M. officinalis, as demonstrated in the study, could potentially be linked to the inhibition of HMG-CoA reductase enzyme expression and a decrease in the expression of SREBP-1c and related genes that contribute to fatty acid syntheses such as acetyl-CoA carboxylase (ACC1), stearoyl-CoA desaturase 1 (SCD), and fatty acid synthase (FAS).30 Given the inconsistencies in findings from research on M. officinalis and lipid profiles in diabetic individuals, further clinical trials are needed to provide a comprehensive understanding. Regarding the effect of saffron and M. officinalis on renal function in individuals with type 2 diabetes, our findings indicated that mean levels of blood urea nitrogen (BUN) and creatinine decreased in the saffron-treated group, but these changes were not statistically significant. This finding is consistent with other studies. Although the effect of saffron extract on renal function has rarely been investigated in human studies, a recent animal investigation demonstrated a substantial decrease in BUN and creatinine in diabetic rats following the administration of crocin (derived from saffron).31
It is believed that the antioxidant properties of crocin contribute to its protective effect on renal function, as suggested by the study authors.31 Additionally, in the current study, there was a significant increase in blood urea and creatinine in M. officinalis group compared to the placebo group. In contrast to our research findings, a study conducted by Zarei et al discovered that M. officinalis extract improves renal performance in diabetic rats by reducing urea and creatinine levels.32 Another study showed that M. officinalis extract has renal protective effects against diabetes and improves renal function in diabetic rats.33 The disparities in these findings can be attributed to differences in research methods and plant dosages. To advance our understanding of type 2 diabetes, more clinical trials are necessary, as previous studies used animal models that may not accurately represent human conditions. Regarding the effect of saffron and M. officinalis on inflammatory factors in individuals with T2DM, our study revealed that saffron does not significantly decrease C-reactive protein (CRP) blood levels, which is consistent with the results of other studies. Additionally, based on the results of the current research, M. officinalis did not noticeably reduce CRP levels in the blood of type 2 diabetes patients. In contrast, Asadi et al found that the consumption of M. officinalis by diabetic patients resulted in a significant decrease in CRP levels.23 According to the systematic review conducted by Zamzuri et al, M. officinalis resulted in considerable changes in hs-CRP.34 This can be attributed to some factors, including variations in sample size, M. officinalis dosage, study duration, and diabetes severity.
Strengths and Limitations
This was the first research examining the effects of M. officinalis and saffron on diabetes, and a comprehensive assessment of patients was carried out at the onset and end of the study. The study faced several limitations, including a small sample size, a short patient follow-up period, and the inability to conduct tests after the study’s termination.
Conclusion
According to the study’s findings, saffron and M. officinalis can reduce FBS and triglycerides in individuals with T2DM, and they can be used alongside conventional medical treatments. Future research should consider a larger sample size and extended duration should be considered for future research studies and should explore the effects of larger doses of saffron and M. officinalis.
Ethics statement
The Ethics Committee of the Tabriz University of Medical Sciences endorsed the study protocol following a thorough evaluation. (Ethical Code: IR.TBZMED.REC.1399.160).
Conflict of interests declaration
The authors declare no conflict of interests.
Data availability statement
The data generated and analyzed in the study are fully presented in this published article.
Author contributions
Conceptualization: Behnam sadighi, Mostafa Araj-Khodaei.
Data curation: Somaiyeh Taheri-Targhi.
Formal analysis: Sarvin Sanaie.
Funding acquisition: Mostafa Araj-Khodaei.
Investigation: Zahra Yousefi,Mostafa Araj-Khodaei.
Methodology: Seyed Kazem Shakouri, Majid Mobasseri.
Supervision: Mostafa Araj-Khodaei.
Validation: Majid Mobasseri.
Writing–original draft: Behnam Sadighi,Zahra Yousefi, Mostafa Araj-Khodaei.
Writing–review & editing: Reza Yarani, Somaiyeh Taheri-Targhi, Sarvin Sanaie.
Consent for publication
Not applicable.
References
-
American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care 2016;39 Suppl 1:S13-22. doi: 10.2337/dc16-S005.
- Badran M, Laher I. Type II diabetes mellitus in Arabic-speaking countries. Int J Endocrinol 2012; 2012:902873. doi: 10.1155/2012/902873 [Crossref] [ Google Scholar]
- Fareed M, Salam N, Khoja AT, Mahmoud MA, Ahamed M. Life style related risk factors of type 2 diabetes mellitus and its increased prevalence in Saudi Arabia: a brief review. Int J Med Res Health Sci 2017; 6(3):125-32. [ Google Scholar]
- Adab Z, Eghtesadi S, Vafa MR, Heydari I, Shojaii A, Haqqani H. Effect of turmeric on glycemic status, lipid profile, hs-CRP, and total antioxidant capacity in hyperlipidemic type 2 diabetes mellitus patients. Phytother Res 2019; 33(4):1173-81. doi: 10.1002/ptr.6312 [Crossref] [ Google Scholar]
- Thomas GN, Jiang CQ, Taheri S, Xiao ZH, Tomlinson B, Cheung BM. A systematic review of lifestyle modification and glucose intolerance in the prevention of type 2 diabetes. Curr Diabetes Rev 2010; 6(6):378-87. doi: 10.2174/157339910793499092 [Crossref] [ Google Scholar]
- Huang Y, Ogurtsova K, Makaroff L, Cavan D, Cho NH. IDF Diabetes Atlas estimates for the global diabetes prevalence of adults aged 18 to 99 years. Diabetes 2017; 66:A446. [ Google Scholar]
- Yu X, Xu L, Zhou Q, Wu S, Tian J, Piao C. The efficacy and safety of the Chinese herbal formula, JTTZ, for the treatment of type 2 diabetes with obesity and hyperlipidemia: a multicenter randomized, positive-controlled, open-label clinical trial. Int J Endocrinol 2018; 2018:9519231. doi: 10.1155/2018/9519231 [Crossref] [ Google Scholar]
- Moravej Aleali A, Amani R, Shahbazian H, Namjooyan F, Latifi SM, Cheraghian B. The effect of hydroalcoholic saffron (Crocus sativus L) extract on fasting plasma glucose, HbA1c, lipid profile, liver, and renal function tests in patients with type 2 diabetes mellitus: a randomized double-blind clinical trial. Phytother Res 2019; 33(6):1648-57. doi: 10.1002/ptr.6351 [Crossref] [ Google Scholar]
- Hosseinzadeh H. Saffron: a herbal medicine of third millennium. Jundishapur J Nat Pharm Prod 2014; 9(1):1-2. doi: 10.17795/jjnpp-16700 [Crossref] [ Google Scholar]
- Modabbernia A, Sohrabi H, Nasehi AA, Raisi F, Saroukhani S, Jamshidi A. Effect of saffron on fluoxetine-induced sexual impairment in men: randomized double-blind placebo-controlled trial. Psychopharmacology (Berl) 2012; 223(4):381-8. doi: 10.1007/s00213-012-2729-6 [Crossref] [ Google Scholar]
- Rooshenas F, Ashrafi M, Nazifi S, Aminlari M, Talebanzadeh S. Evaluation the effect of saffron aqueous extract on oxidative stress parameters and important biochemical enzymes of liver tissue in streptozotocin-induced diabetic rats. J Arak Univ Med Sci 2018;21(5):77-87. [Persian].
- Kianbakht S, Hajiaghaee R. Anti-hyperglycemic effects of saffron and its active constituents, crocin and safranal, in alloxan-induced diabetic rats. J Med Plants 2011; 10(39):82-9. [ Google Scholar]
- Khodsook S, Moshtaghian J. Effect extract of Melissa officinalis on blood lipids and lipoproteins and the prevention of diabetes in rats. Iranian Journal of Diabetes and Metabolism 2015;14(5):315-24. [Persian].
- Shakeri A, Sahebkar A, Javadi B. Melissa officinalis L - A review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol 2016; 188:204-28. doi: 10.1016/j.jep.2016.05.010 [Crossref] [ Google Scholar]
- Al-Ishaq RK, Abotaleb M, Kubatka P, Kajo K, Büsselberg D. Flavonoids and their anti-diabetic effects: cellular mechanisms and effects to improve blood sugar levels. Biomolecules 2019; 9(9):430. doi: 10.3390/biom9090430 [Crossref] [ Google Scholar]
- Sarian MN, Ahmed QU, Mat So’ad SZ, Alhassan AM, Murugesu S, Perumal V. Antioxidant and antidiabetic effects of flavonoids: a structure-activity relationship-based study. Biomed Res Int 2017; 2017:8386065. doi: 10.1155/2017/8386065 [Crossref] [ Google Scholar]
- Kwon YI, Vattem DA, Shetty K. Evaluation of clonal herbs of Lamiaceae species for management of diabetes and hypertension. Asia Pac J Clin Nutr 2006; 15(1):107-18. [ Google Scholar]
- Miroliaei M, Khazaei S, Moshkelgosha S, Shirvani M. Inhibitory effects of lemon balm (Melissa officinalis, L) extract on the formation of advanced glycation end products. Food Chem 2011; 129(2):267-71. doi: 10.1016/j.foodchem.2011.04.039 [Crossref] [ Google Scholar]
- Parham M, Bagherzadeh M, Asghari M, Akbari H, Hosseini Z, Rafiee M. Evaluating the effect of a herb on the control of blood glucose and insulin-resistance in patients with advanced type 2 diabetes (a double-blind clinical trial). Caspian J Intern Med 2020; 11(1):12-20. doi: 10.22088/cjim.11.1.12 [Crossref] [ Google Scholar]
- Senadheera SP, Ekanayake S, Wanigatunge C. Anti-hyperglycaemic effects of herbal porridge made of Scoparia dulcis leaf extract in diabetics - a randomized crossover clinical trial. BMC Complement Altern Med 2015; 15:410. doi: 10.1186/s12906-015-0935-6 [Crossref] [ Google Scholar]
- Khalili N, Fereydoonzadeh R, Mohtashami R, Mehrzadi S, Heydari M, Fallah Huseini H. Silymarin, olibanum, and nettle, a mixed herbal formulation in the treatment of type II diabetes: a randomized, double-blind, placebo-controlled, clinical trial. J Evid Based Complementary Altern Med 2017; 22(4):603-8. doi: 10.1177/2156587217696929 [Crossref] [ Google Scholar]
- Nasimi Doost Azgomi R, Karimi A, Zarshenas MM, Moini Jazani A. The mechanisms of saffron (Crocus sativus L) on the inflammatory pathways of diabetes mellitus: a systematic review. Diabetes Metab Syndr 2022; 16(1):102365. doi: 10.1016/j.dsx.2021.102365 [Crossref] [ Google Scholar]
- Asadi A, Shidfar F, Safari M, Hosseini AF, Fallah Huseini H, Heidari I. Efficacy of Melissa officinalis L (lemon balm) extract on glycemic control and cardiovascular risk factors in individuals with type 2 diabetes: a randomized, double-blind, clinical trial. Phytother Res 2019; 33(3):651-9. doi: 10.1002/ptr.6254 [Crossref] [ Google Scholar]
- Chung MJ, Cho SY, Bhuiyan MJ, Kim KH, Lee SJ. Anti-diabetic effects of lemon balm (Melissa officinalis) essential oil on glucose- and lipid-regulating enzymes in type 2 diabetic mice. Br J Nutr 2010; 104(2):180-8. doi: 10.1017/s0007114510001765 [Crossref] [ Google Scholar]
- Asadi A, Shidfar F, Safari M, Malek M, Hosseini AF, Rezazadeh S. Safety and efficacy of Melissa officinalis (lemon balm) on ApoA-I, Apo B, lipid ratio and ICAM-1 in type 2 diabetes patients: a randomized, double-blinded clinical trial. Complement Ther Med 2018; 40:83-8. doi: 10.1016/j.ctim.2018.07.015 [Crossref] [ Google Scholar]
- Karimi-Nazari E, Nadjarzadeh A, Masoumi R, Marzban A, Mohajeri SA, Ramezani-Jolfaie N. Effect of saffron (Crocus sativus L) on lipid profile, glycemic indices and antioxidant status among overweight/obese prediabetic individuals: a double-blinded, randomized controlled trial. Clin Nutr ESPEN 2019; 34:130-6. doi: 10.1016/j.clnesp.2019.07.012 [Crossref] [ Google Scholar]
- Barari A, Amini S, Mansouri B. The effect of saffron extract and aerobic training on serum HbA1C and Apo A1 in men with diabetes mellitus, type II diabetes. Jundishapur J Physiol 2018; 1(2):e148602. [ Google Scholar]
- Tajaddini A, Roshanravan N, Mobasseri M, Haleem Al-Qaim Z, Hadi A, Aeinehchi A. The effect of saffron (Crocus sativus L) on glycemia, lipid profile, and antioxidant status in patients with type-2 diabetes mellitus: a randomized placebo-controlled trial. Phytother Res 2023; 37(2):388-98. doi: 10.1002/ptr.7600 [Crossref] [ Google Scholar]
- Asbaghi O, Soltani S, Norouzi N, Milajerdi A, Choobkar S, Asemi Z. The effect of saffron supplementation on blood glucose and lipid profile: a systematic review and meta-analysis of randomized controlled trials. Complement Ther Med 2019; 47:102158. doi: 10.1016/j.ctim.2019.07.017 [Crossref] [ Google Scholar]
- Jun HJ, Lee JH, Jia Y, Hoang MH, Byun H, Kim KH. Melissa officinalis essential oil reduces plasma triglycerides in human apolipoprotein E2 transgenic mice by inhibiting sterol regulatory element-binding protein-1c-dependent fatty acid synthesis. J Nutr 2012; 142(3):432-40. doi: 10.3945/jn.111.152538 [Crossref] [ Google Scholar]
- Naghizadeh B, Mansouri SM, Vahdati Mashhadian N. Crocin attenuates cisplatin-induced renal oxidative stress in rats. Food Chem Toxicol 2010; 48(10):2650-5. doi: 10.1016/j.fct.2010.06.035 [Crossref] [ Google Scholar]
- Rezaei Kelishadi M, Zamani-Doabi S, Ghasemi A, Rahimi A, Nabi N, Changizi-Ashtiyani S, et al. The effects of hydroalcoholic extract of Melissa officinalis L. on the level of renal function and liver enzymes in diabetic rats. Iranian Journal of Endocrinology and Metabolism 2016;17(5):353-61. [Persian].
- Nasiri AR, Karami-Bonari AR. Protective effects of Melissa officinalis L. extract on gentamicin-induced renal failure in diabetic rats. Med Lab J 2023;17(1):35-41. [Persian].
- Zamzuri MA, Mansor J, Nurumal SR, Jamhari MN, Arifin MA, Nawi AM. Herbal antioxidants as tertiary prevention against cardiovascular complications in type 2 diabetes mellitus: a systematic review. J Herb Med 2023; 37:100621. doi: 10.1016/j.hermed.2022.100621 [Crossref] [ Google Scholar]