Int J Drug Res Clin. 2:e28.
doi: 10.34172/ijdrc.2024.e28
Review Article
Long-Term Outcomes and Potential Late-Onset Side Effects of Combination Therapy in Older Immunocompromised Patients Treated for COVID-19
Mohammad Amin Akbarzadeh 1  , Mahsa Akbarzadeh 1
, Mahsa Akbarzadeh 1  , Yosra Vaez-Gharamaleki 2
, Yosra Vaez-Gharamaleki 2  , Mohammad-Salar Hosseini 3, 4, *
, Mohammad-Salar Hosseini 3, 4, * 
Author information:
1Research Center for Evidence-Based Medicine, Iranian EBM Center: A JBI Center of Excellence, Tabriz University of Medical Sciences, Tabriz, Iran
2Hematology and Oncology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
3Research Center for Integrative Medicine in Aging, Aging Research Institute, Tabriz University of Medical Sciences, Tabriz, Iran
4Medical Philosophy and History Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
The COVID-19 pandemic has affected global health, particularly vulnerable populations such as the aged and immunocompromised individuals who face heightened risks due to age-related physiological changes and comorbidities. Recent cohort studies have indicated that older adults constitute a substantial proportion of hospitalizations and mortality associated with COVID-19, due to decreased physiological reserves and immunosenescence. Combination therapy has been proposed as a primary therapeutic strategy for managing severe COVID-19 in immunocompromised patients, typically involving multiple pharmacological agents, including antivirals, immunomodulatory agents, and supportive care. Compared to monotherapy regimens, recent studies have demonstrated that combination therapies lead to reduced viral load, improved clinical outcomes, and lower mortality rates. Meanwhile, the long-term outcomes of these combination therapies, along with specific details of the post-acute sequelae of COVID-19 (PASC), also referred to as long COVID, in older immunocompromised patients remain under investigation. This review aims to explore these aspects in greater detail, investigating the efficacy of combination therapies in treating COVID-19 among older immunocompromised patients, with a particular focus on their long-term implications.
Keywords: Aged, Combined modality therapy, COVID-19, Immunocompromised, Post-acute COVID-19 syndrome
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
None.
Please cite this article as follows: Akbarzadeh MA, Akbarzadeh M, Vaez-Gharamaleki Y, Hosseini MS. Long-term outcomes and potential late-onset side effects of combination therapy in older immunocompromised patients treated for COVID-19. Int J Drug Res Clin. 2024; 2: e28. doi: 10.34172/ijdrc.2024.e28
Introduction
The COVID-19 pandemic has transformed the landscape of global health, presenting unprecedented challenges, particularly for vulnerable populations such as the elderly and the immunocompromised individuals.1 Immunocompromised patients, including those with a history of recent or ongoing cancer, HIV/AIDS infection, or advanced chronic conditions, are at a higher risk of complications.2,3 The situation is further complicated by the fact that older adults often suffer from multiple chronic conditions, making the management of COVID-19 in this demographic particularly challenging. This population, already susceptible to a range of infectious diseases, has faced disproportionate risks from COVID-19.4
The gravity of COVID-19’s impact on the older adults can be observed in mortality statistics. Studies show that adults over 65 years old account for approximately 80% of hospitalizations and face a significantly higher risk of death from the disease compared to younger populations.5 This susceptibility is primarily due to reduced physiological reserves and common comorbidities such as cardiovascular disease, diabetes, and chronic respiratory diseases, which are prevalent in this age group.6 Furthermore, immunosenescence, associated with aging, impairs the body’s ability to respond effectively to infections, thereby complicating the course and consequences of the disease.7
Combination therapy has been proposed as a primary therapeutic strategy for managing severe COVID-19 in older immunocompromised patients.8 Typically, combination therapy involves the use of multiple pharmacological agents, including antivirals, immunomodulatory agents, and supportive care, aimed at targeting various aspects of the virus’s life cycle and the host’s immune response.9 The rationale behind this approach is to enhance therapeutic efficacy by using drugs that act on different viral targets or host responses, potentially reducing viral load and ameliorating symptoms more effectively than monotherapy. However, while combination therapy may provide immediate benefits in reducing morbidity and mortality, it also introduces complexities associated with polypharmacy and the concurrent use of multiple medications by patients.10
Moreover, the long-term effects of these therapies are not adequately understood. Preliminary reports and ongoing studies suggest the potential for long-term organ damage resulting from both the virus and the intensive drug regimens used in treatment, particularly affecting the kidneys, liver, and cardiovascular system.11 For instance, antivirals such as remdesivir, which has been widely used during the pandemic, have been associated with renal and liver toxicity in some patients.12 Similarly, although steroids like dexamethasone can reduce mortality in critically ill patients, they also pose risks such as hyperglycemia, osteoporosis, and increased susceptibility to other infections—complications that are particularly problematic in aged patients.13 The use of immunomodulators such as tocilizumab, which has been administered to suppress the immune system’s overactivation, also illustrates the delicate balance required in treating COVID-19.14
This review aimed to explore these issues in depth, examining the efficacy and safety of combination therapies in treating COVID-19 among elderly immunocompromised patients, with a particular focus on the long-term implications of these therapeutic strategies.
Combination Therapy Strategies
Combination therapy for COVID-19 typically involves using multiple pharmacological agents that target different stages of the SARS-CoV-2 life cycle. In older immunocompromised patients, the rationale for combination therapy is to achieve a synergistic or additive effect that can lead to a more robust antiviral response, potentially overcoming the compromised immune system’s inability to effectively control the virus.15 Figure 1 presents a step-wise approach to COVID-19 in aged immunocompromised patients.
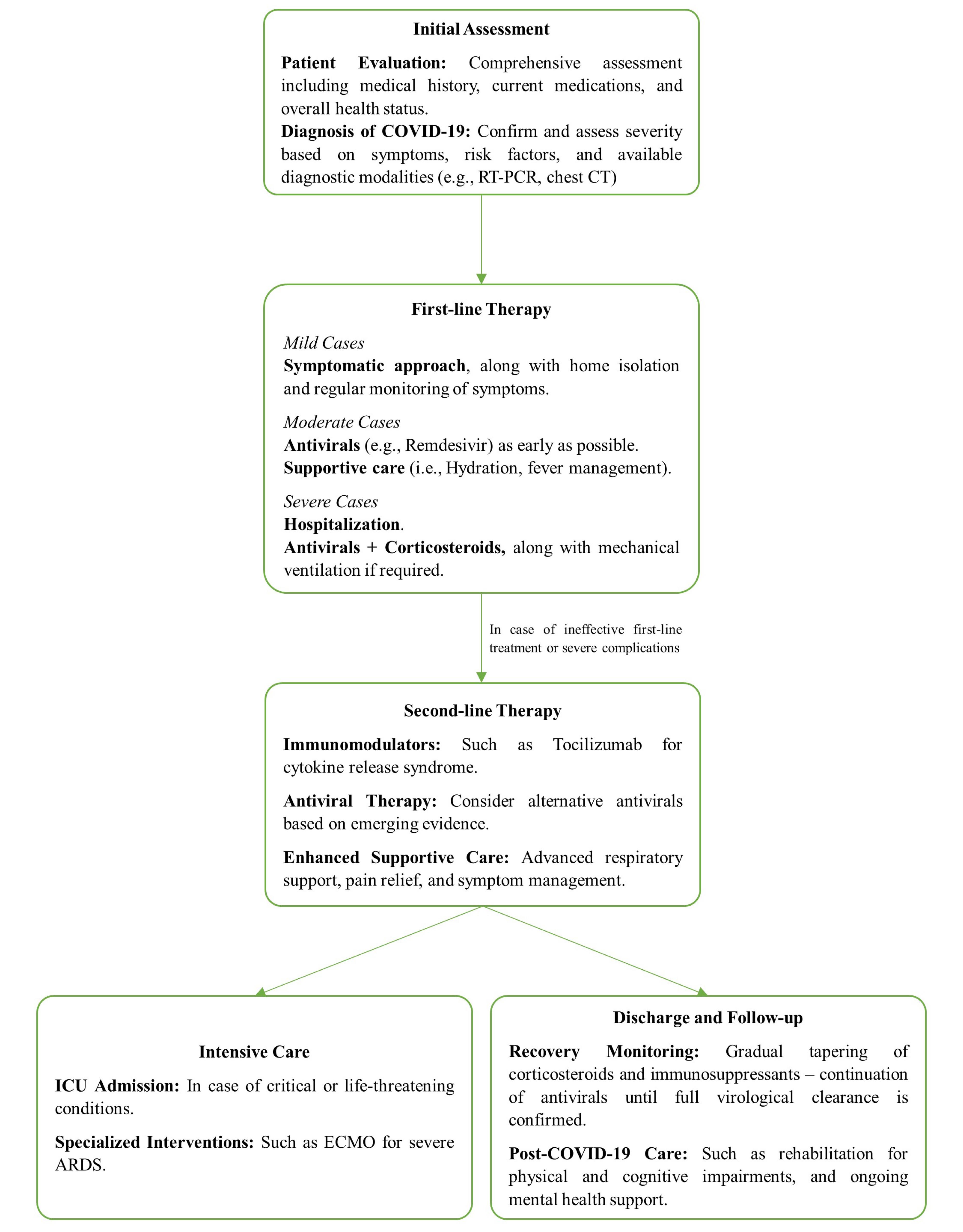
Figure 1.
Stepwise Approach to Older Immunocompromised Adults with COVID-19. Note. ARDS: Acute respiratory distress syndrome; CT: Computed tomography; ECMO: Extracorporeal membrane oxygenation; ICU: Intensive care unit; RT-PCR: Reverse transcription polymerase chain reaction.
.
Stepwise Approach to Older Immunocompromised Adults with COVID-19. Note. ARDS: Acute respiratory distress syndrome; CT: Computed tomography; ECMO: Extracorporeal membrane oxygenation; ICU: Intensive care unit; RT-PCR: Reverse transcription polymerase chain reaction.
The agents used in combination therapy for COVID-19 may include direct-acting antivirals such as remdesivir, which inhibit viral RNA-dependent RNA polymerase, protease inhibitors like nirmatrelvir, which suppress the viral replication, immunomodulators such as the Janus kinase inhibitor baricitinib, and monoclonal antibodies that target the spike protein, preventing viral entry into host cells.16-20
Several studies have evaluated the efficacy of combination therapy in aged immunocompromised patients. Recent studies have focused on the effectiveness of combination treatments involving remdesivir, nirmatrelvir/ritonavir, and monoclonal antibodies such as tixagevimab/cilgavimab and sotrovimab in immunocompromised patients with persistent COVID-19.8,21,22 However, a subset of patients exhibited only partial responses, indicating the need for further research to optimize treatment strategies.23
These studies have generally demonstrated that combination therapy can lead to reduced viral load as measured by RT-PCR, shortened time to clinical improvement, decreased length of hospitalization, and lowered mortality rates compared to monotherapy or standard care.24,25 Table 1 summarizes the prominent proposed combination therapy strategies.
Table 1.
The Proposed Combination Therapy Strategies for Older and Immunocompromised COVID-19 Patients
|
Authors
|
Year
|
Country
|
Study Design
|
Intervention
|
Outcomes
|
| Chen et al (BLAZE-1 Trial Group)26 |
2022 |
USA |
RCT |
Bamlanivimab and etesevimab |
Patients receiving a combination of bamlanivimab and etesevimab experienced significantly faster resolution of symptoms, along with, a lower rate of COVID-19-related hospitalization. |
| Lowe et al (FLARE Investigators)27 |
2022 |
UK |
RCT |
Lopinavir/ritonavir and favipiravir |
A non-significant reduction in viral load was observed compared to placebo, and significantly more participants in this arm achieved undetectable virus levels on day five. However, the lopinavir/ritonavir treatment showed no statistically significant change in viral load reduction compared to placebo. |
| Arabi et al28 |
2021 |
International multicenter |
RCT |
Lopinavir/ritonavir and hydroxychloroquine |
The intervention group exhibited decreased OSF days and reduced hospital survival compared to the control group. |
| Fumeaux et al (REMAP-CAP Investigators)29 |
2023 |
Switzerland |
RCT |
Pamapimod and pioglitazone |
The proportion of patients alive and without oxygen or respiratory support during the 28-day treatment period and those requiring invasive MV were not significantly different. Mortality rates were also not significantly different. |
Kalil et al
(ACTT-2 Study Group Members)30 |
2021 |
International multicenter |
RCT |
Remdesivir and baricitinib |
A decreased recovery time was observed in the combination group, with a non-significantly lower 28-day mortality rate compared to the control group (5.1% vs 7.8%). |
| Polizzotto et al (ITAC INSIGHT 013 Study Group)31 |
2022 |
International multicenter |
RCT |
Remdesivir and hyperimmune immunoglobulin |
The hyperimmune immunoglobulin group did not show significantly greater odds of a more favorable outcome by day 7. |
| Kalil et al (ACTT-3 study group members)32 |
2021 |
International multicenter |
RCT |
Remdesivir and IFN-β-1a |
The mean duration of symptoms did not significantly differ among study groups. The estimated 28-day mortality rate was higher in the IFN-β-1a + remdesivir group compared to the placebo + remdesivir group (5% vs 3%), which was not statistically significant. |
| Marrone et al33 |
2022 |
Italy |
Quasi-experimental study |
Remdesivir and dexamethasone |
A significantly increased viral clearance was observed in the remdesivir/dexamethasone group compared to dexamethasone alone (median 6 vs 16 days). Additionally, the 30-day mortality rate and length of hospital stay were significantly lower in the remdesivir/dexamethasone group compared to dexamethasone alone. |
| Malhani et al34 |
2021 |
Saudi Arabia |
Cohort study |
Lopinavir/ritonavir, ribavirin, and IFN-β-1a |
IFN-based therapy was associated with lower 28-day mortality as compared to favipiravir. No differences were observed in the length of hospital stay. The IFN-treated group also required significantly less use of systemic corticosteroids compared to the favipiravir group. |
| Ader et al35 |
2021 |
France |
RCT |
IFN-β-1a, hydroxychloroquine, and lopinavir/ritonavir |
No significant effect of investigational treatment was observed on SARS-CoV-2 clearance. |
Note. IFN-β: Interferon beta; MV: Mechanical ventilation; OSF: Organ support-free; RCT: Randomized controlled trial.
Combination therapy is typically more aggressive in aged immunocompromised patients due to their reduced natural immune response. It appears to be most effective when initiated early in the disease course.23,36 Moreover, there is a lower incidence of treatment-resistant variations in patients treated with combination therapy. Some studies suggest that combination therapy may help in ‘priming’ of immune system, leading to a more effective immune response upon subsequent exposure to the virus.37
Nutritional interventions have been a vital component of combination therapy strategies since the beginning of the pandemic, owing to their availability, low cost, and minimal side effects.38 Dietary supplements such as vitamin C, vitamin D, selenium, zinc, probiotics, and omega-3 fatty acids, particularly eicosapentaenoic acid and docosahexaenoic acid, have been widely studied for their immunomodulatory and anti-inflammatory effects.39-43 Implementing targeted nutritional interventions into combination therapy regimens could contribute to an improved approach to COVID-19 management in this vulnerable population, potentially enhancing treatment outcomes and long-term recovery.44
Vaccination also remains a cornerstone in the prevention of COVID-19. Immunocompromised individuals are advised to receive additional vaccine doses for better protection.45 Monoclonal antibodies such as pemivibart, have recently received emergency use authorization for immunocompromised patients who may not respond sufficiently to vaccination.46 However, pemivibart is not a substitute for vaccination.
Mechanisms of Adverse Effects
The biochemical and physiological mechanisms underlying the observed drug interactions and side effects in older immunocompromised patients treated with combination therapy for COVID-19 are complex and multifactorial.
Many antiviral and immunomodulatory drugs used in combination therapy regimens are metabolized by the hepatic cytochrome P450 enzyme system.47 For example, remdesivir, a commonly used antiviral for COVID-19, is metabolized through this pathway.48 Concurrent administration of drugs that inhibit or induce these enzymes can alter drug levels, potentially leading to toxicity or reduced efficacy. For instance, the co-administration of remdesivir with a potent CYP3A4 inhibitor such as ketoconazole may increase remdesivir levels, enhancing the risk of renal toxicity.49 On the other hand, pharmacodynamic interactions occur when drugs affect the same physiological system but act at different sites or through different mechanisms. For instance, using corticosteroids along with nonsteroidal anti-inflammatory drugs (NSAIDs) in COVID-19 patients can increase the risk of gastrointestinal bleeding, a serious side effect given the complementary effects of these drugs on the integrity and function of gastrointestinal mucosa.50 Additionally, polypharmacy is a significant concern due to the high prevalence of chronic illnesses that require ongoing pharmacological management.51 Interactions between various medications can lead to adverse drug reactions, which in turn may result in hospital admissions, increased healthcare costs, and overall poorer health outcomes.
Pathophysiological Implications of Long-term Effects
Renal Impairment
Drugs such as remdesivir are known to cause acute kidney injury in some patients. The mechanism involves drug-induced nephrotoxicity, possibly exacerbated by COVID-19-related systemic inflammation and hypoxia, which further compromises renal microcirculation.52 In aged patients, pre-existing reduced renal function compounds this risk, necessitating careful monitoring and dosage adjustments.
Hepatic Stress
Antivirals can also impose a significant burden on liver function. The metabolism of these drugs produces reactive metabolites that can cause oxidative stress and inflammation in hepatocytes.53,54 Chronic administration can lead to a buildup of these metabolites, potentially resulting in drug-induced liver injury.54 Monitoring liver enzyme levels in patients receiving long-term antiviral therapy is crucial to detect early signs of liver stress or damage.
Cardiovascular Effects
The interaction of COVID-19 with cardiovascular medications is particularly concerning. COVID-19 can induce myocardial injury through direct viral invasion and systemic inflammation.55 When combined with drugs such as hydroxychloroquine – initially considered in COVID-19 treatment – which can cause prolonged QT interval, there is an increased risk of arrhythmias.56 This effect is particularly dangerous for older adults, who may have pre-existing heart conditions.
Follow-up studies have shown that patients who recover from COVID-19, particularly those who experienced severe symptoms requiring hospitalization, may continue to exhibit signs of cardiac impairment, such as reduced ejection fraction or ongoing myocardial inflammation, detectable via echocardiography and cardiac magnetic resonance imaging (MRI).57 These findings suggest a direct connection between the disease and enduring cardiac changes, likely exacerbated by aggressive pharmacological treatment during the acute phase.
Neurological Sequels
The neurotropic nature of SARS-CoV-2 suggests that it can directly invade nervous system tissues, leading to neurological symptoms.58 The long-term use of systemic corticosteroids, often necessary in severe COVID-19 cases, can exacerbate these issues, leading to mood disorders, insomnia, and even cognitive decline in susceptible individuals.59 These agents cross the blood-brain barrier and can alter neurotransmitter function, contributing to these neuropsychiatric effects.
There is emerging evidence suggesting that survivors of severe COVID-19, particularly those treated in intensive care units (ICUs), may experience long-term cognitive decline, a condition some researchers are beginning to refer to as COVID-19 brain fog.60 This condition includes a range of symptoms, including memory loss, difficulty concentrating, and even changes in executive function.61 The exact mechanism behind these symptoms is not fully understood but is believed to involve both direct viral effects and the consequences of prolonged hospitalization, including the use of sedatives and paralytics that can impact brain function.62
Psychological Consequences
Beyond the physical impacts, the long-term psychological consequences of COVID-19 and its treatments cannot be overlooked. The stress of illness, combined with isolation during hospital stays, can exacerbate or trigger new-onset mental health issues such as anxiety, depression, and post-traumatic stress disorder. The role of medications, particularly those affecting the central nervous system, in managing COVID-19 adds complexity to this situation. Benzodiazepines, occasionally used for their sedative properties during intensive care, can lead to dependence and withdrawal issues that may persist well after recovery from the acute illness.
Frailty and Sarcopenia
Prolonged immobility during hospitalization, coupled with the catabolic effects of corticosteroids, can lead to significant muscle wasting and weakness, a condition termed ICU-acquired weakness (ICUAW).63,64 This condition can severely impact the quality of life (QoL), resulting in prolonged physical rehabilitation needs and, in some cases, permanent disability.65 Older patients are particularly susceptible to these effects due to their reduced baseline muscle mass and strength.66,67
Long-term Outcomes
The long-term outcomes of combination therapy in older immunocompromised patients are still under evaluation.68,69 Studies have reported that early intervention with combination therapy is associated with better outcomes, including complete virological clearance in some cases. In contrast, lower response rates and higher disease severity were observed when treatment is initiated later.70 Long-term outcomes should be evaluated in three main domains:
Clinical and Virological Outcomes
Achieving a sustained virological response is a key indicator of the efficacy of combination therapy in the context of COVID-19, particularly for immunocompromised patients who may have a reduced antiviral immune response.21 Long-term follow-up is essential to monitor symptoms of COVID-19 recurrence or reinfection since these patients may be at an increased risk for these events due to their underlying conditions.71
Post-acute sequelae of COVID-19 (PASC), also known as Long COVID or Post-COVID-19 syndrome, refers to a range of symptoms that persist for weeks to months after the acute phase of the infection has resolved.72,73 In older immunocompromised patients, the risk factors and pathophysiology of PASC may differ, and the impact of combination therapy on the incidence and severity of PASC is not yet fully understood.74,75
Additionally, the inappropriate use of antibiotics in COVID-19 patients could lead to devastating complications of antimicrobial resistance, which plays a significant role in morbidity and mortality worldwide – especially in immunocompromised patients.76,77 Apart from the coinfection and superinfection, improper administration of antibiotics increases the long-term risk of antimicrobial resistance, leading to increased mortality risk in this population.78
Functional and Quality-of-Life Outcomes
Assessments of mobility, activities of daily living, and the need for rehabilitation services are the most important indicators of elderly functional capacity. Furthermore, health-related QoL encompasses the physical, psychological, and social domains of health.79 It is a broad measure that reflects the patient’s perceived well-being and ability to carry out daily activities.80
Although studies have not observed a significant difference in the prevalence of PACS among immunocompromised and non-immunocompromised patients so far,81 aged patients are at a significantly higher risk of developing PASC.82 With most studies still ongoing, current evidence from the latest studies suggests the significant effect of PASC on health-related QoL in terms of fatigue, pain, low physical activity, cognitive, and psychological issues among aged patients.83,84 Further long-term studies are required to evaluate how the health-related QoL is affected by treatment regimens in this population, particularly focusing on the persistence of symptoms such as fatigue, cognitive impairment, and psychological distress.
Immunological Outcomes
In immunocompromised patients, the reconstitution of the immune system post-treatment is a complex process that may be influenced by factors such as age, the nature of the immunocompromising condition, and the specific agents used in combination therapy.85 The risk of autoimmune phenomena or hyperinflammatory syndromes following combination therapy is a concern, especially in patients with pre-existing autoimmune conditions or those receiving immunomodulatory drugs as part of their treatment regimen.86 Monitoring the recovery and function of immune cells over time is essential to understand the long-term immunological impact of COVID-19 and its treatment.
Future Directions
Immunocompromised patients may not develop effective humoral or cell-mediated immunity in response to natural infection or vaccination.87 This complicates the assessment of clinical deterioration and patient-oriented treatment approaches. Additionally, the timing of treatment initiation is critical as early combination therapy has shown better outcomes.70 Using multiple drugs targeting different viral proteins and mechanisms may enhance efficacy, especially in the absence of humoral immunity; however, the optimal duration and combination of these treatments still need to be determined through controlled trials.88 Close monitoring of immunocompromised individuals is essential until complete viral clearance is achieved as they may act as a reservoir for novel viral variants.89 Moreover, the impact of combination therapy on hospitalization and mortality rates needs to be closely observed in this population.
Prospective studies should compare early single-drug therapy to early combination regimens and include a longer follow-up to evaluate the long-term safety and efficacy of these treatments. Longitudinal cohort studies with regular follow-up visits and assessments are invaluable for tracking the long-term outcomes of combination therapy in older immunocompromised patients. These studies can provide data on symptom persistence, the incidence of PASC, and the long-term safety and efficacy of treatment regimens. Further controlled trials are also needed to establish the optimal treatment for persistent COVID-19 in this patient population. As we get deeper insights into PASC and post-COVID-19 syndrome, it is essential to define optimal management strategies for these conditions.
Conclusion
Managing COVID-19 in older immunocompromised patients requires a comprehensive approach beyond immediate clinical care. Considering the long-term health trajectories of patients, the potential cumulative effects of their existing medications, and the novel COVID-19 treatments, a personalized and patient-oriented approach is essential for this population. Moreover, immunosenescence and altered immune response complicate the treatment landscape further. Ongoing monitoring is crucial for managing the long-term risks associated with combination therapy. This includes regular evaluations of kidney and liver function, cardiovascular health, and neurological status. Monitoring should be proactive and frequent enough to detect changes early, allowing timely interventions to mitigate adverse effects. Reevaluating the treatment regimen is equally important as new studies emerge and our understanding of the long-term impacts of COVID-19, PASC, and their treatments evolves. Treatment plans should be dynamic, adapting to the latest clinical guidelines, research findings, and the patient’s health status. Regular follow-ups and reassessments provide opportunities to deprescribe unnecessary medications and reduce polypharmacy, thereby minimizing the risk of drug interactions and side effects.
Ethics statement
Not applicable.
Conflict of interests declaration
The authors declare no conflict of interests.
Acknowledgements
We appreciate the support provided by the Clinical Research Development Unit, Imam Reza General Hospital (Tabriz, Iran) in conducting this research.
Data availability statement
This article contains all data produced or analyzed during this investigation.
Author contributions
Conceptualization: Mohammad Amin Akbarzadeh, Mohammad-Salar Hosseini.
Data curation: Mohammad Amin Akbarzadeh, Mahsa Akbarzadeh.
Investigation: Mohammad Amin Akbarzadeh, Mahsa Akbarzadeh.
Visualization: Yosra Vaez-Gharamaleki, Mohammad-Salar Hosseini.
Writing–original draft: Mohammad Amin Akbarzadeh, Mahsa Akbarzadeh.
Writing–review & editing: Yosra Vaez-Gharamaleki, Mohammad-Salar Hosseini.
Consent for publication
Not applicable.
References
- Akbarzadeh MA, Hosseini MS. Implications for cancer care in Iran during COVID-19 pandemic. Radiother Oncol 2020; 148:211-2. doi: 10.1016/j.radonc.2020.04.041 [Crossref] [ Google Scholar]
- Akbarzadeh MA, Vaez-Gharamaleki Y, Jahanshahlou F, Ghaffari Bavil A, Hamzehzadeh S, Seifimansour S. Outcomes of COVID-19 infection in patients with chronic lymphocytic leukemia: a systematic review and meta-analysis. Rev Assoc Med Bras 2024; 70:e20240322. doi: 10.1590/1806-9282.20240322 [Crossref] [ Google Scholar]
- Danwang C, Noubiap JJ, Robert A, Yombi JC. Outcomes of patients with HIV and COVID-19 co-infection: a systematic review and meta-analysis. AIDS Res Ther 2022; 19(1):3. doi: 10.1186/s12981-021-00427-y [Crossref] [ Google Scholar]
- Couture S, Lepage MA, Godard-Sebillotte C, Sourial N, Talbot-Hamon C, Kremer R. Geriatric syndromes in older adults hospitalized with COVID-19 in Montreal, Canada. Can Geriatr J 2022; 25(3):269-78. doi: 10.5770/cgj.25.579 [Crossref] [ Google Scholar]
- Mueller AL, McNamara MS, Sinclair DA. Why does COVID-19 disproportionately affect older people?. Aging (Albany NY) 2020; 12(10):9959-81. doi: 10.18632/aging.103344 [Crossref] [ Google Scholar]
- Farshbafnadi M, Kamali Zonouzi S, Sabahi M, Dolatshahi M, Aarabi MH. Aging & COVID-19 susceptibility, disease severity, and clinical outcomes: the role of entangled risk factors. Exp Gerontol 2021; 154:111507. doi: 10.1016/j.exger.2021.111507 [Crossref] [ Google Scholar]
- Akbarzadeh MA, Hosseini MS. Is COVID-19 really a geriatric syndrome?. Ageing Res Rev 2022; 79:101657. doi: 10.1016/j.arr.2022.101657 [Crossref] [ Google Scholar]
- Focosi D, Maggi F, D’Abramo A, Nicastri E, Sullivan DJ. Antiviral combination therapies for persistent COVID-19 in immunocompromised patients. Int J Infect Dis 2023; 137:55-9. doi: 10.1016/j.ijid.2023.09.021 [Crossref] [ Google Scholar]
- Akinbolade S, Coughlan D, Fairbairn R, McConkey G, Powell H, Ogunbayo D. Combination therapies for COVID-19: an overview of the clinical trials landscape. Br J Clin Pharmacol 2022; 88(4):1590-7. doi: 10.1111/bcp.15089 [Crossref] [ Google Scholar]
- Chang TI, Park H, Kim DW, Jeon EK, Rhee CM, Kalantar-Zadeh K. Polypharmacy, hospitalization, and mortality risk: a nationwide cohort study. Sci Rep 2020; 10(1):18964. doi: 10.1038/s41598-020-75888-8 [Crossref] [ Google Scholar]
- Li J, Zhou Y, Ma J, Zhang Q, Shao J, Liang S. The long-term health outcomes, pathophysiological mechanisms and multidisciplinary management of long COVID. Signal Transduct Target Ther 2023; 8(1):416. doi: 10.1038/s41392-023-01640-z [Crossref] [ Google Scholar]
- Blair HA. Remdesivir: a review in COVID-19. Drugs 2023; 83(13):1215-37. doi: 10.1007/s40265-023-01926-0 [Crossref] [ Google Scholar]
- Alessi J, de Oliveira GB, Schaan BD, Telo GH. Dexamethasone in the era of COVID-19: friend or foe? An essay on the effects of dexamethasone and the potential risks of its inadvertent use in patients with diabetes. Diabetol Metab Syndr 2020; 12:80. doi: 10.1186/s13098-020-00583-7 [Crossref] [ Google Scholar]
- Mohammed MA. Fighting cytokine storm and immunomodulatory deficiency: by using natural products therapy up to now. Front Pharmacol 2023; 14:1111329. doi: 10.3389/fphar.2023.1111329 [Crossref] [ Google Scholar]
- Shyr ZA, Cheng YS, Lo DC, Zheng W. Drug combination therapy for emerging viral diseases. Drug Discov Today 2021; 26(10):2367-76. doi: 10.1016/j.drudis.2021.05.008 [Crossref] [ Google Scholar]
- Gordon CJ, Tchesnokov EP, Woolner E, Perry JK, Feng JY, Porter DP. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J Biol Chem 2020; 295(20):6785-97. doi: 10.1074/jbc.RA120.013679 [Crossref] [ Google Scholar]
- Hashemian SM, Sheida A, Taghizadieh M, Memar MY, Hamblin MR, Bannazadeh Baghi H. Paxlovid (nirmatrelvir/ritonavir): a new approach to COVID-19 therapy?. Biomed Pharmacother 2023; 162:114367. doi: 10.1016/j.biopha.2023.114367 [Crossref] [ Google Scholar]
- Cox M, Peacock TP, Harvey WT, Hughes J, Wright DW, Willett BJ. SARS-CoV-2 variant evasion of monoclonal antibodies based on in vitro studies. Nat Rev Microbiol 2023; 21(2):112-24. doi: 10.1038/s41579-022-00809-7 [Crossref] [ Google Scholar]
- Tahsini Tekantapeh S, Ghojazadeh M, Ghamari AA, Mohammadi A, Soleimanpour H. Therapeutic and anti-inflammatory effects of baricitinib on mortality, ICU transfer, clinical improvement, and CRS-related laboratory parameters of hospitalized patients with moderate to severe COVID-19 pneumonia: a systematic review and meta-analysis. Expert Rev Respir Med 2022; 16(10):1109-32. doi: 10.1080/17476348.2022.2114899 [Crossref] [ Google Scholar]
- Morgan MS, Yan K, Le TT, Johnston RA, Amarilla AA, Muller DA. Monoclonal antibodies specific for SARS-CoV-2 spike protein suitable for multiple applications for current variants of concern. Viruses 2022; 15(1):139. doi: 10.3390/v15010139 [Crossref] [ Google Scholar]
- Brosh-Nissimov T, Ma’aravi N, Leshin-Carmel D, Edel Y, Ben Barouch S, Segman Y. Combination treatment of persistent COVID-19 in immunocompromised patients with remdesivir, nirmaltrevir/ritonavir and tixegavimab/cilgavimab. J Microbiol Immunol Infect 2024; 57(1):189-94. doi: 10.1016/j.jmii.2023.09.004 [Crossref] [ Google Scholar]
- Scotto R, Buonomo AR, Iuliano A, Foggia M, Sardanelli A, Villari R. Remdesivir alone or in combination with monoclonal antibodies as an early treatment to prevent severe COVID-19 in patients with mild/moderate disease at high risk of progression: a single centre, real-life study. Vaccines (Basel) 2023; 11(2):200. doi: 10.3390/vaccines11020200 [Crossref] [ Google Scholar]
- Orth HM, Flasshove C, Berger M, Hattenhauer T, Biederbick KD, Mispelbaum R. Early combination therapy of COVID-19 in high-risk patients. Infection 2024; 52(3):877-89. doi: 10.1007/s15010-023-02125-5 [Crossref] [ Google Scholar]
- Hirai J, Mori N, Sakanashi D, Ohashi W, Shibata Y, Asai N. Real-world experience of the comparative effectiveness and safety of combination therapy with remdesivir and monoclonal antibodies versus remdesivir alone for patients with mild-to-moderate COVID-19 and immunosuppression: a retrospective single-center study in Aichi, Japan. Viruses 2023; 15(9):1952. doi: 10.3390/v15091952 [Crossref] [ Google Scholar]
- Lingas G, Néant N, Gaymard A, Belhadi D, Peytavin G, Hites M. Effect of remdesivir on viral dynamics in COVID-19 hospitalized patients: a modelling analysis of the randomized, controlled, open-label DisCoVeRy trial. J Antimicrob Chemother 2022; 77(5):1404-12. doi: 10.1093/jac/dkac048 [Crossref] [ Google Scholar]
- Chen P, Behre G, Hebert C, Kumar P, Farmer Macpherson L, Graham-Clarke PL. Bamlanivimab and etesevimab improve symptoms and associated outcomes in ambulatory patients at increased risk for severe coronavirus disease 2019: results from the placebo-controlled double-blind phase 3 BLAZE-1 trial. Open Forum Infect Dis 2022; 9(5):ofac172. doi: 10.1093/ofid/ofac172 [Crossref] [ Google Scholar]
- Lowe DM, Brown LK, Chowdhury K, Davey S, Yee P, Ikeji F. Favipiravir, lopinavir-ritonavir, or combination therapy (FLARE): a randomised, double-blind, 2 × 2 factorial placebo-controlled trial of early antiviral therapy in COVID-19. PLoS Med 2022; 19(10):e1004120. doi: 10.1371/journal.pmed.1004120 [Crossref] [ Google Scholar]
- Arabi YM, Gordon AC, Derde LP, Nichol AD, Murthy S, Beidh FA. Lopinavir-ritonavir and hydroxychloroquine for critically ill patients with COVID-19: REMAP-CAP randomized controlled trial. Intensive Care Med 2021; 47(8):867-86. doi: 10.1007/s00134-021-06448-5 [Crossref] [ Google Scholar]
- Fumeaux T, Berger C, Bausch A, Wright M, Vilimanovich U, Soldatovic I. The KINETIC phase 2 randomized controlled trial of oral pamapimod-pioglitazone in non-critically ill COVID-19 inpatients. iScience 2023; 26(10):108038. doi: 10.1016/j.isci.2023.108038 [Crossref] [ Google Scholar]
- Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med 2021; 384(9):795-807. doi: 10.1056/NEJMoa2031994 [Crossref] [ Google Scholar]
- ITAC (INSIGHT 013) Study Group. Hyperimmune immunoglobulin for hospitalised patients with COVID-19 (ITAC): a double-blind, placebo-controlled, phase 3, randomised trial. Lancet 2022; 399(10324):530-40. doi: 10.1016/s0140-6736(22)00101-5 [Crossref] [ Google Scholar]
- Kalil AC, Mehta AK, Patterson TF, Erdmann N, Gomez CA, Jain MK. Efficacy of interferon beta-1a plus remdesivir compared with remdesivir alone in hospitalised adults with COVID-19: a double-bind, randomised, placebo-controlled, phase 3 trial. Lancet Respir Med 2021; 9(12):1365-76. doi: 10.1016/s2213-2600(21)00384-2 [Crossref] [ Google Scholar]
- Marrone A, Nevola R, Sellitto A, Cozzolino D, Romano C, Cuomo G. Remdesivir plus dexamethasone versus dexamethasone alone for the treatment of coronavirus disease 2019 (COVID-19) patients requiring supplemental O2 therapy: a prospective controlled nonrandomized study. Clin Infect Dis 2022; 75(1):e403-9. doi: 10.1093/cid/ciac014 [Crossref] [ Google Scholar]
- Malhani AA, Enani MA, Saheb Sharif-Askari F, Alghareeb MR, Bin-Brikan RT, AlShahrani SA. Combination of (interferon beta-1b, lopinavir/ritonavir and ribavirin) versus favipiravir in hospitalized patients with non-critical COVID-19: a cohort study. PLoS One 2021; 16(6):e0252984. doi: 10.1371/journal.pone.0252984 [Crossref] [ Google Scholar]
- Ader F, Peiffer-Smadja N, Poissy J, Bouscambert-Duchamp M, Belhadi D, Diallo A. An open-label randomized controlled trial of the effect of lopinavir/ritonavir, lopinavir/ritonavir plus IFN-β-1a and hydroxychloroquine in hospitalized patients with COVID-19. Clin Microbiol Infect 2021; 27(12):1826-37. doi: 10.1016/j.cmi.2021.05.020 [Crossref] [ Google Scholar]
- Scaglione V, Rotundo S, Marascio N, De Marco C, Lionello R, Veneziano C. Lessons learned and implications of early therapies for coronavirus disease in a territorial service centre in the Calabria region: a retrospective study. BMC Infect Dis 2022; 22(1):793. doi: 10.1186/s12879-022-07774-9 [Crossref] [ Google Scholar]
- Zhand S, Saghaeian Jazi M, Mohammadi S, Tarighati Rasekhi R, Rostamian G, Kalani MR. COVID-19: the immune responses and clinical therapy candidates. Int J Mol Sci 2020; 21(15):5559. doi: 10.3390/ijms21155559 [Crossref] [ Google Scholar]
- Sanaie S. Dietary supplements: boon or bane?. nt J Drug Res Clin 2023; 1(1):e16. doi: 10.34172/ijdrc.2023.e16 [Crossref] [ Google Scholar]
- Balboni E, Zagnoli F, Filippini T, Fairweather-Tait SJ, Vinceti M. Zinc and selenium supplementation in COVID-19 prevention and treatment: a systematic review of the experimental studies. J Trace Elem Med Biol 2022; 71:126956. doi: 10.1016/j.jtemb.2022.126956 [Crossref] [ Google Scholar]
- Balafar M, Mahmoodpoor A, Arjmandi H, Maddah Khelejani A, Soleimanpour H. High-dose vitamin C in the treatment of COVID-19 patients in intensive care unit; a letter to the editor. Arch Acad Emerg Med 2024; 12(1):e41. doi: 10.22037/aaem.v12i1.2233 [Crossref] [ Google Scholar]
- Nikniaz L, Akbarzadeh MA, Hosseinifard H, Hosseini MS. The impact of vitamin D supplementation on mortality rate and clinical outcomes of COVID-19 patients: a systematic review and meta-analysis. Pharm Sci 2021; 27(Suppl 1):S1-12. doi: 10.34172/ps.2021.13 [Crossref] [ Google Scholar]
- Nursyifa Fadiyah N, Megawati G, Erlangga Luftimas D. Potential of omega 3 supplementation for coronavirus disease 2019 (COVID-19): a scoping review. Int J Gen Med 2022; 15:3915-22. doi: 10.2147/ijgm.s357460 [Crossref] [ Google Scholar]
- Mahmoodpoor A, Shamekh A, Sanaie S. A debate on vitamin C: supplementation on the hotline for critically ill patients with COVID-19. Adv Pharm Bull 2021; 11(3):395-6. doi: 10.34172/apb.2021.046 [Crossref] [ Google Scholar]
- Altooq N, Humood A, Alajaimi A, Alenezi AF, Janahi M, AlHaj O. The role of micronutrients in the management of COIVD-19 and optimizing vaccine efficacy. Hum Nutr Metab 2022; 27:200141. doi: 10.1016/j.hnm.2022.200141 [Crossref] [ Google Scholar]
- Parker EP, Desai S, Marti M, Nohynek H, Kaslow DC, Kochhar S. Response to additional COVID-19 vaccine doses in people who are immunocompromised: a rapid review. Lancet Glob Health 2022; 10(3):e326-8. doi: 10.1016/s2214-109x(21)00593-3 [Crossref] [ Google Scholar]
- Focosi D, Franchini M, Casadevall A, Maggi F. An update on the anti-spike monoclonal antibody pipeline for SARS-CoV-2. Clin Microbiol Infect 2024; 30(8):999-1006. doi: 10.1016/j.cmi.2024.04.012 [Crossref] [ Google Scholar]
- Stavropoulou E, Pircalabioru GG, Bezirtzoglou E. The role of cytochromes P450 in infection. Front Immunol 2018; 9:89. doi: 10.3389/fimmu.2018.00089 [Crossref] [ Google Scholar]
- Deb S, Reeves AA, Hopefl R, Bejusca R. ADME and pharmacokinetic properties of remdesivir: its drug interaction potential. Pharmaceuticals (Basel) 2021; 14(7):655. doi: 10.3390/ph14070655 [Crossref] [ Google Scholar]
- Patanwala AE, Jager NG, Radosevich JJ, Brüggemann R. An update on drug-drug interactions for care of the acutely ill in the era of COVID-19. Am J Health Syst Pharm 2023; 80(19):1301-8. doi: 10.1093/ajhp/zxad152 [Crossref] [ Google Scholar]
- Sohail R, Mathew M, Patel KK, Reddy SA, Haider Z, Naria M. Effects of non-steroidal anti-inflammatory drugs (NSAIDs) and gastroprotective NSAIDs on the gastrointestinal tract: a narrative review. Cureus 2023; 15(4):e37080. doi: 10.7759/cureus.37080 [Crossref] [ Google Scholar]
- Sharifi A, Jahanshahlou F, Gol Mohammadi Senji A, Hamzezadeh S, Zarei M, Hosseini MS. Enhanced recovery after surgery protocols and procedures in geriatric surgery. Int J Aging 2024; 2(1):e2. doi: 10.34172/ija.2024.e2 [Crossref] [ Google Scholar]
- Shams G, Kazemi A, Jafaryan K, Morowvat MH, Peymani P, Karimzadeh I. Acute kidney injury in COVID-19 patients receiving remdesivir: a systematic review and meta-analysis of randomized clinical trials. Clinics (Sao Paulo) 2023; 78:100200. doi: 10.1016/j.clinsp.2023.100200 [Crossref] [ Google Scholar]
- Allison R, Guraka A, Shawa IT, Tripathi G, Moritz W, Kermanizadeh A. Drug induced liver injury - a 2023 update. J Toxicol Environ Health B Crit Rev 2023; 26(8):442-67. doi: 10.1080/10937404.2023.2261848 [Crossref] [ Google Scholar]
- Villanueva-Paz M, Morán L, López-Alcántara N, Freixo C, Andrade RJ, Lucena MI. Oxidative stress in drug-induced liver injury (DILI): from mechanisms to biomarkers for use in clinical practice. Antioxidants (Basel) 2021; 10(3):390. doi: 10.3390/antiox10030390 [Crossref] [ Google Scholar]
- Saed Aldien A, Ganesan GS, Wahbeh F, Al-Nassr N, Altarawneh H, Al Theyab L. Systemic inflammation may induce cardiac injury in COVID-19 patients including children and adolescents without underlying cardiovascular diseases: a systematic review. Cardiovasc Revasc Med 2022; 35:169-78. doi: 10.1016/j.carrev.2021.04.007 [Crossref] [ Google Scholar]
- Hooks M, Bart B, Vardeny O, Westanmo A, Adabag S. Effects of hydroxychloroquine treatment on QT interval. Heart Rhythm 2020; 17(11):1930-5. doi: 10.1016/j.hrthm.2020.06.029 [Crossref] [ Google Scholar]
- Paruchuri SS, Farwa UE, Jabeen S, Pamecha S, Shan Z, Parekh R. Myocarditis and myocardial injury in long COVID syndrome: a comprehensive review of the literature. Cureus 2023; 15(7):e42444. doi: 10.7759/cureus.42444 [Crossref] [ Google Scholar]
- Veleri S. Neurotropism of SARS-CoV-2 and neurological diseases of the central nervous system in COVID-19 patients. Exp Brain Res 2022; 240(1):9-25. doi: 10.1007/s00221-021-06244-z [Crossref] [ Google Scholar]
- Foroughi M, Gupta R, Ganguly A, Mirza J, Fotros A. Neuropsychiatric manifestations of COVID-19: a review. Adv Psychiatry Behav Health 2021; 1(1):161-72. doi: 10.1016/j.ypsc.2021.05.003 [Crossref] [ Google Scholar]
- Sia AL, Neo JE, Jen-Wei Tan B, Tan EK. “Brain fog” and COVID-19. Am J Med Sci 2023; 365(5):472-4. doi: 10.1016/j.amjms.2023.01.003 [Crossref] [ Google Scholar]
- Nouraeinejad A. Brain fog as a long-term sequela of COVID-19. SN Compr Clin Med 2023; 5(1):9. doi: 10.1007/s42399-022-01352-5 [Crossref] [ Google Scholar]
- Leng A, Shah M, Ahmad SA, Premraj L, Wildi K, Li Bassi G. Pathogenesis underlying neurological manifestations of long COVID syndrome and potential therapeutics. Cells 2023; 12(5):816. doi: 10.3390/cells12050816 [Crossref] [ Google Scholar]
- Jolley SE, Bunnell AE, Hough CL. ICU-acquired weakness. Chest 2016; 150(5):1129-40. doi: 10.1016/j.chest.2016.03.045 [Crossref] [ Google Scholar]
- Qin ES, Hough CL, Andrews J, Bunnell AE. Intensive care unit-acquired weakness and the COVID-19 pandemic: a clinical review. PM R 2022; 14(2):227-38. doi: 10.1002/pmrj.12757 [Crossref] [ Google Scholar]
- Intiso D, Centra AM, Bartolo M, Gatta MT, Gravina M, Di Rienzo F. Recovery and long-term functional outcome in people with critical illness polyneuropathy and myopathy: a scoping review. BMC Neurol 2022; 22(1):50. doi: 10.1186/s12883-022-02570-z [Crossref] [ Google Scholar]
- Rahiminezhad E, Zakeri MA, Dehghan M. Muscle strength/intensive care unit acquired weakness in COVID-19 and non-COVID-19 patients. Nurs Crit Care 2023; 28(6):1012-21. doi: 10.1111/nicc.12830 [Crossref] [ Google Scholar]
- Yamada K, Kitai T, Iwata K, Nishihara H, Ito T, Yokoyama R. Predictive factors and clinical impact of ICU-acquired weakness on functional disability in mechanically ventilated patients with COVID-19. Heart Lung 2023; 60:139-45. doi: 10.1016/j.hrtlng.2023.03.008 [Crossref] [ Google Scholar]
- Yadav BS. High-dose methotrexate and zanubrutinib combination therapy for primary central nervous system lymphoma. World J Clin Oncol 2024; 15(3):371-4. doi: 10.5306/wjco.v15.i3.371 [Crossref] [ Google Scholar]
- Khadela A, Soni S, Megha K, Bhagat S, Chavda VP. A review on the impact of the SARS-CoV-2 omicron subvariant on elderly patients with diverse co-morbidities. Biologics 2023; 3(2):138-57. doi: 10.3390/biologics3020008 [Crossref] [ Google Scholar]
- Gentile I, Foggia M, Silvitelli M, Sardanelli A, Cattaneo L, Viceconte G. Optimizing COVID-19 treatment in immunocompromised patients: early combination therapy with remdesivir, nirmatrelvir/ritonavir and sotrovimab. Virol J 2023; 20(1):301. doi: 10.1186/s12985-023-02269-8 [Crossref] [ Google Scholar]
- DeWolf S, Laracy JC, Perales MA, Kamboj M, van den Brink MRM, Vardhana S. SARS-CoV-2 in immunocompromised individuals. Immunity 2022; 55(10):1779-98. doi: 10.1016/j.immuni.2022.09.006 [Crossref] [ Google Scholar]
- Proal AD, VanElzakker MB. Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol 2021; 12:698169. doi: 10.3389/fmicb.2021.698169 [Crossref] [ Google Scholar]
- Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS. Post-acute COVID-19 syndrome. Nat Med 2021; 27(4):601-15. doi: 10.1038/s41591-021-01283-z [Crossref] [ Google Scholar]
- Baker SJ, Nfonsam LE, Leto D, Rutherford C, Smieja M, McArthur AG. Chronic COVID-19 infection in an immunosuppressed patient shows changes in lineage over time: a case report. Virol J 2024; 21(1):8. doi: 10.1186/s12985-023-02278-7 [Crossref] [ Google Scholar]
- Dioverti V, Salto-Alejandre S, Haidar G. Immunocompromised patients with protracted COVID-19: a review of “long persisters”. Curr Transplant Rep 2022; 9(4):209-18. doi: 10.1007/s40472-022-00385-y [Crossref] [ Google Scholar]
- Zeshan B, Karobari MI, Afzal N, Siddiq A, Basha S, Basheer SN. The usage of antibiotics by COVID-19 patients with comorbidities: the risk of increased antimicrobial resistance. Antibiotics (Basel) 2021; 11(1):35. doi: 10.3390/antibiotics11010035 [Crossref] [ Google Scholar]
- Jahanshahlou F, Hosseini MS. Antibiotic resistance: a disregarded concern for misuse of azithromycin in COVID-19 treatment. J Res Med Sci 2021; 26:101. doi: 10.4103/jrms.JRMS_1124_20 [Crossref] [ Google Scholar]
- Dueñas D, Daza J, Liscano Y. Coinfections and superinfections associated with COVID-19 in Colombia: a narrative review. Medicina (Kaunas) 2023; 59(7):1336. doi: 10.3390/medicina59071336 [Crossref] [ Google Scholar]
- Krawczyk-Suszek M, Kleinrok A. Health-related quality of life (HRQoL) of people over 65 years of age. Int J Environ Res Public Health 2022; 19(2):625. doi: 10.3390/ijerph19020625 [Crossref] [ Google Scholar]
- Sitlinger A, Zafar SY. Health-related quality of life: the impact on morbidity and mortality. Surg Oncol Clin N Am 2018; 27(4):675-84. doi: 10.1016/j.soc.2018.05.008 [Crossref] [ Google Scholar]
- Román-Montes CM, Flores-Soto Y, Guaracha-Basañez GA, Tamez-Torres KM, Sifuentes-Osornio J, González-Lara MF. Post-COVID-19 syndrome and quality of life impairment in severe COVID-19 Mexican patients. Front Public Health 2023; 11:1155951. doi: 10.3389/fpubh.2023.1155951 [Crossref] [ Google Scholar]
- Cohen K, Ren S, Heath K, Dasmariñas MC, Jubilo KG, Guo Y. Risk of persistent and new clinical sequelae among adults aged 65 years and older during the post-acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ 2022; 376:e068414. doi: 10.1136/bmj-2021-068414 [Crossref] [ Google Scholar]
- Shanbehzadeh S, Zanjari N, Yassin M, Yassin Z, Tavahomi M. Association between long COVID, functional activity, and health-related quality of life in older adults. BMC Geriatr 2023; 23(1):40. doi: 10.1186/s12877-023-03757-w [Crossref] [ Google Scholar]
- Mansell V, Hall Dykgraaf S, Kidd M, Goodyear-Smith F. Long COVID and older people. Lancet Healthy Longev 2022; 3(12):e849-54. doi: 10.1016/s2666-7568(22)00245-8 [Crossref] [ Google Scholar]
- Goldman JD, Robinson PC, Uldrick TS, Ljungman P. COVID-19 in immunocompromised populations: implications for prognosis and repurposing of immunotherapies. J Immunother Cancer 2021; 9(6):e002630. doi: 10.1136/jitc-2021-002630 [Crossref] [ Google Scholar]
- Sher EK, Ćosović A, Džidić-Krivić A, Farhat EK, Pinjić E, Sher F. COVID-19 a triggering factor of autoimmune and multi-inflammatory diseases. Life Sci 2023; 319:121531. doi: 10.1016/j.lfs.2023.121531 [Crossref] [ Google Scholar]
- Baldi F, Dentone C, Mikulska M, Fenoglio D, Mirabella M, Magnè F. Case report: sotrovimab, remdesivir and nirmatrelvir/ritonavir combination as salvage treatment option in two immunocompromised patients hospitalized for COVID-19. Front Med (Lausanne) 2022; 9:1062450. doi: 10.3389/fmed.2022.1062450 [Crossref] [ Google Scholar]
- Helleberg M, Niemann CU, Moestrup KS, Kirk O, Lebech AM, Lane C. Persistent COVID-19 in an immunocompromised patient temporarily responsive to two courses of remdesivir therapy. J Infect Dis 2020; 222(7):1103-7. doi: 10.1093/infdis/jiaa446 [Crossref] [ Google Scholar]
- Ghafari M, Hall M, Golubchik T, Ayoubkhani D, House T, MacIntyre-Cockett G. Prevalence of persistent SARS-CoV-2 in a large community surveillance study. Nature 2024; 626(8001):1094-101. doi: 10.1038/s41586-024-07029-4 [Crossref] [ Google Scholar]