Int J Drug Res Clin. 2:e18.
doi: 10.34172/ijdrc.2024.e18
Original Article
Different Concentrations of Produced Recombinant TNF-α Protein Caused Different Cellular Viability in Breast Cancer Cell Lines
Sara Tirabadi 1  , Mohammad Reza Nahai 2, Nazila Alizadeh 1, Mahya Ahmadpour Youshanlui 1, Behzad Baradaran 1, 3, *
, Mohammad Reza Nahai 2, Nazila Alizadeh 1, Mahya Ahmadpour Youshanlui 1, Behzad Baradaran 1, 3, * 
Author information:
1Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2Department of Microbiology, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
3Departemnt of Immunology, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Background:
Breast cancer is a prevalent malignancy among women globally. The implementation of early detection methods and improved treatment strategies have shown a considerable decrease in the financial burden associated with breast cancer mortality. One of the inflammatory cytokines is tumor necrosis factor (TNF), produced during chronic inflammation, apoptosis, tumor proliferation, and angiogenesis. The main objectives of this study were to produce recombinant TNF-α protein and assess its impact on the MCF-7 and MDA-MB-468 cancer cell lines.
Methods:
The present study employed the SHuffle® T7 express strain of E. coli to synthesize recombinant TNF-α protein, primarily due to its advantageous characteristics of cost-effectiveness and high productivity. The molecular weight of the protein was determined using SDS-PAGE and Coomassie blue staining techniques, and its presence was subsequently verified through Western blot analysis. Subsequently, the cells were treated with the produced recombinant protein, and the cellular viability was assessed using the MTT assay. Then, the Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) technique was employed to quantify the expression level of the Caspase-3 gene, known as an apoptotic factor, and the interleukin-1 (IL-1) gene, recognized as a pro-inflammatory factor, in both cell lines.
Results:
Lower quantities of recombinant TNF-α protein were shown to cause cellular cytotoxicity, according to the findings of the MTT test, while cellular proliferation was observed at increased levels of recombinant TNF-α protein. The results of the qRT-PCR test exhibited a lack of identifiable patterns in both cell lines.
Conclusion:
The findings indicate that cellular viability is significantly decreased at a minimal concentration of 5-10 ng/mL of TNF-α, whereas it is noticeably elevated at concentrations higher than 10 ng/mL. Furthermore, gene expression analysis revealed a significant reduction in IL-1 expression in both cell lines after administering a high dose of TNF-α.
Keywords: Breast cancer, Recombinant TNF-α protein, E. coli, Cell viability
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
This work received no financial support.
Please cite this article as follows: Tirabadi S, Nahai MR, Alizadeh N, Ahmadpour Youshanlui M, Baradaran B. Different concentrations of produced recombinant TNF-α protein caused different cellular viability in breast cancer cell lines. Int J Drug Res Clin. 2024; 2: e18. doi: 10.34172/ijdrc.2024.e18
Introduction
Breast cancer has emerged as the prevailing form of malignancy in females on a global scale. According to the available data, it is the second most prevalent cause of mortality.1,2 The annual incidence of cancer is estimated to be approximately 2 million cases.3 Within the scope of clinical practices, breast cancer is commonly regarded as a complex disease that can be categorized into various subgroups. These subcategories consist of Luminal A, Luminal B, Luminal-Her2, Her2-positive, Triple-negative (TNBC), and Triple-negative basal-like/non-basal. Each subgroup has unique molecular characteristics, therapeutic choices, and prognostic results.4 Over the last several decades, scientists have delved deeply into the underlying causes of breast cancer, with a particular focus on two molecular phenomena: genetic alterations and epigenetic modifications.5,6 Within the tumor microenvironment (TME), there is a complex interplay between healthy cells and the extracellular matrix that envelops malignant cells.7 Cancer cells have the capability to target and eliminate various healthy cells present in the stroma and immune system, promoting their proliferation and invasion.8-10 Numerous inflammatory cytokines contribute to the development, invasion, and metastasis stages, as well as to the regulation of various proteolytic enzymes.11 Tumor necrosis factor-alpha (TNF-α) is well acknowledged as an inflammatory cytokine that performs an essential function in promoting the upregulation of proteases linked to cancer invasion and metastasis. This inflammatory cytokine is secreted not only by immune cells such as macrophages and lymphocytes but also by non-immune cells such as tumor cells. Notably, TNF possesses the ability to induce structural alterations in certain cell types, with a primary focus on tumor cells.12,13 Both tumor cells and stromal cells possess the capability to secrete chemokines, leading to the infiltration of monocytes and subsequent differentiation into macrophages.14,15 The two primary states of macrophages, namely the classically active state (M1) and the alternatively activated state (M2), are commonly known as tumor-associated macrophages. In the breast cancer TME, TNF-α plays a vital role as a pro-inflammatory cytokine. It is predominantly produced by stromal cells, specifically M1 tumor-associated macrophages, along with cancer cells within the TME. TNF-α showcases various cellular functions, encompassing apoptotic activity and the promotion of cancer cell growth, proliferation, angiogenesis, invasion, and migration. Through its interaction with TNFR1 and TNFR2 receptors, TNF-α can regulate cellular signaling pathways effectively.16 The administration of exogenous TNF-α at the local level has been demonstrated to promote the degradation of the vasculature surrounding tumors, leading to the subsequent necrosis of tumor cells through indirect methods. However, it is crucial to emphasize that the cytotoxic impact of TNF-α is contingent on the presence of additional metabolic inhibitors.17,18 The majority of research on TNF-α and tumors focuses on soluble TNF-α (sTNF-α), which is a prevalent pro-inflammatory cytokine. As a result, sTNF-α has a major role in establishing the link between inflammation and cancer progression.19 Furthermore, it should be noted that the refolding procedures employed may not achieve complete restoration of the native protein fold, potentially leading to a decrease in the functionality of recombinant TNF-α. The SHuffle® T7 Express strain of E. coli, genetically engineered by New England Biolabs in the United States, presents a valuable resource for protein expression. This strain is particularly useful for proteins that require disulfide bonds for proper folding and functionality. Additionally, it possesses the ability to rectify mis-oxidized bonds and promote efficient protein production.20,21 In the present study, recombinant TNF-α was generated using E. coli SHuffle® T7 Express. Assessing the impact of TNF-α on breast cancer cell lines was the primary objective of this study. The correlation between the expression of apoptosis-associated proteins and an adverse prognosis in breast cancer is intricately linked to caspase-3 22. Another important inflammatory mediator cytokine involved in the mechanisms of carcinogenesis and tumor advancement is interleukin-1 (IL-1).23 The second objective of the research was to find out the ways the two factors were implicated in carcinogenesis, namely Caspase-3 and IL-1, with the presence of TNF-α protein.
Methods
Bacterial Strain
In this investigation, the E. coli SHuffle® T7 Express strain was chosen as the primary host for expressing the soluble TNF-α protein. This strain has been genetically engineered to enhance the expression of proteins containing disulfide bonds, making it well-suited for producing properly folded and biologically active TNF-α. The SHuffle® T7 Express strain contains genes encoding disulfide isomerases that aid in the correct folding of proteins, including TNF-α. This approach offers a cost-effective and efficient means to study the function and potential therapeutic applications of TNF-α in various cellular processes, such as carcinogenesis and tumor progression.
Expression of Large-Scale Protein
The pET-28a ( + ) expression vector, which contains a 6X His tag, was utilized for the purpose of achieving significant overexpression of the recombinant protein of TNF-α. For achieving the intended objective, the pTZ57R/T vector, which harbors the sTNF-α gene, and the pET-28a expression vector were both subjected to digestion using the restriction enzymes NcoI and XhoI. After digesting the vectors, they were purified using a gel, followed by ligation of the insert fragment into the linearized pET-28a ( + ) expression vector, specifically between the NcoI and XhoI restriction sites. The resulting ligation product was introduced into chemically competent E. coli SHuffle® T7 Express cells (New England Biolabs, USA) and subsequently cultured on LB agar plates treated with kanamycin (50 μg/mL), following the prescribed protocol for the SHuffle® T7 Express strain. The mature colonies went through additional analysis utilizing the specific PCR primers designed for the T7 promoter. The recombinant plasmid was inserted into a newly transformed strain of E. coli SHuffle® T7 Express. The strain was later propagated in 10 mL of 2XTY medium containing 16 g Bacto Tryptone, 10 g Bacto Yeast Extract, and 5 g NaCl. A pH of 7.0 was achieved in the medium, and the final volume was made up to 1 L using distilled water. The manufacturing process was carried out under controlled conditions at a temperature of 30 °C for an extended duration using an orbital shaker. The stimulation of protein synthesis was accomplished by supplementing the solution with 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG), leading to the preparation of the desired final concentration. Afterwards, the mixture went through an incubation period for an additional duration of 6 hours at a temperature of 30 °C. Subsequently, the cellular specimens were collected through centrifugation at 5000 g for 30 minutes. Finally, the liquid component of the sample, known as the supernatant, was discarded, while the solid components, referred to as pellets, were subjected to freezing at a temperature of -20 °C.
Extraction and Purification of Recombinant TNF-α Protein
The bacterial pellets of E. coli obtained from the culture were reconstituted using a lysis buffer with the purpose of expediting subsequent procedures. The lysis buffer was prepared by combining sodium dihydrogen phosphate (50 mM), sodium chloride (300 mM), and imidazole (10 mM) at a pH of 8. In order to optimize the lysis process, lysozyme was added to the lysis buffer at a concentration of 1 mg/mL. For achieving comprehensive disruption of the bacterial cells and optimizing the extraction of cellular contents, the suspension was exposed to sonication. The process of sonication was repeated six times, wherein each iteration encompassed a series of brief pulses lasting for a duration of 30 seconds. A sonication power of 150 W was utilized during the procedure. After each pulse, a 30-second interval was implemented to mitigate the risk of excessive heat accumulation and to ensure the sustained efficacy of sonication. Efficient cell lysis and release of desired target molecules for subsequent analysis or downstream applications were achieved through the synergistic effects of the lysis buffer composition, supplemented with lysozyme, and the repeated cycles of sonication.
Ion Affinity Chromatography
The generated TNF-α protein was purified using immobilized metal ion affinity chromatography (IMAC) according to the manufacturer’s instructions (Qiagen, Netherlands). The lysate-containing proteins were applied to a column that was filled with immobilized metal ions. Subsequently, a low-concentration imidazole wash buffer was used to remove non-specific contaminants. Subsequently, the purified protein was subjected to a step-wise elution procedure, wherein the imidazole concentration in the elution buffer was gradually increased. Following the purification process, the protein went through dialysis against phosphate-buffered saline (PBS) at a concentration of 1 X for an extended period of time, typically overnight. This dialysis step was performed in order to eliminate any remaining impurities and facilitate the exchange of the buffer solution. The IMAC purification method yielded a purified protein in its native state, which is appropriate for subsequent analysis and experimentation.
SDS-PAGE
The important aim of this study was to evaluate the purity and integrity of the purified recombinant TNF-α protein using the Coomassie blue-stained and SDS-PAGE technique. The protein samples, together with molecular weight markers, were introduced into wells that were formed in the stacking gel. The application of an electric current induced the migration of proteins in the gel, with their movement being influenced by their different sizes. Following the electrophoresis procedure, the gel was subjected to staining using Coomassie blue dye, and any extra dye was subsequently eliminated via destaining. Subsequently, the gel that was first exposed to staining was examined and evaluated with the goal of finding out the dimensions and level of homogeneity of the recombinant TNF-α protein. The SDS-PAGE system offered an accurate method for assessing the quality and integrity of proteins.
Cell Culture
The MCF-7 and MDA-MB-468 cell lines, obtained from the National Cell Bank of Iran, were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and a combination of penicillin and streptomycin (Gibco, Carlsbad, CA, USA). These cells were maintained in a 95% humidified incubator at 37 °C with a 5% CO2 atmosphere, providing an optimized environment for their growth and viability during the experimental study.
Cell Cytotoxicity Assay
The investigation involved the utilization of the MTT assay, which is a colorimetric method employing 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide, to assess the half-maximal inhibitory concentration (IC50) of TNF-α on both the MCF7 and MDA-MB-468 cell lines. Cells were cultured in 96-well plates at a density of 10 × 103 cells per well and allowed to reach 75% confluency within 24 hours. Subsequently, the cells were exposed to varying concentrations of TNF-α (50, 500, 1000, and 10000 ng/mL) for a duration of 24 hours. After this incubation period, the cells were washed and treated with MTT (2 mg/mL) in the culture medium, followed by a 4-hour incubation at 37 °C. To solubilize the formazan crystals formed, 200 μL of dimethyl sulfoxide (DMSO) was added to each well, and after a 30-minute incubation, the absorbance was measured at 570 nm using an ELISA reader (Tecan, Switzerland). The experiment was conducted in triplicate to ensure the reliability and validity of the findings.
RNA Preparation, cDNA Synthesis, and qRT -PCR
The investigation involved the evaluation of gene expression related to apoptosis, specifically caspase-3 and IL-1, through the utilization of Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR). To achieve this objective, the sequential procedures of RNA extraction and cDNA synthesis were conducted in the prescribed order. The RNA extraction was performed using a commercially available kit (BioFact, South Korea). For cDNA synthesis, the cDNA BioFact TM RT kit (BioFact, South Korea) was employed. The cDNA was synthesized using 0.5 µg of RNA extract, 0.1 µg of random primers (Table 1), and 200 U of Moloney murine leukemia virus (M-MLV) reverse transcriptase enzyme, adhering strictly to the manufacturer’s instructions. Subsequently, Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) was conducted using the LightCycler 96 instrument (Roche Diagnostics, Mannheim, Germany). Notably, the experimental process was replicated in triplicate to ensure scientific rigor and reproducibility of outcomes. For data analysis, the 2-ΔΔCT method was employed, a well-recognized technique in the field of gene expression quantification.
Table 1.
Sequences of Primers
|
Sequence of primers
|
Primers
|
Genes
|
| 5′- ATGATGGCTTATTACAGTGGCAA-3′ |
Forward |
IL-1 |
| 5′- GTCGGAGATTCGTAGCTGGA-3′ |
Reverse |
| 5′-TGTCATCTCGCTCTGGTACG-3' |
Forward |
CASP-3 |
| 5′-AAATGACCCCTTCATCACCA-3′ |
Reverse |
| 5′-ACCCGTTGAACCCCATTCGTGA-3′ |
Forward |
18 S |
| 5′-GCCTCACTAAACCATCCAATCGG-3′ |
Reverse |
Statistical Analysis
In this study, each dataset underwent a minimum of three distinct and independent evaluations. The measurement data were utilized to calculate the mean and standard deviation. Group differences and similarities were assessed employing t-tests or analysis of variance (ANOVA). The statistical analysis was conducted using GraphPad Prism version 8 (GraphPad Software, San Diego, California, USA), with a significance level set at P < 0.05.
Results
Expression and Purification of Recombinant TNF-α
The presence of the recombinant TNF-α protein was confirmed through SDS-PAGE analysis following the induction process. Densitometry was utilized to quantify the amount of TNF-α generated by the strain, based on the density of the corresponding bands observed on stained SDS-PAGE gels. Successful purification of the recombinant TNF-α protein was accomplished using Ni-NTA columns in the immobilized metal affinity chromatography (IMAC) technique. The samples collected during column washing with washing buffer were labeled E1 to E7 and further analyzed through SDS-PAGE, confirming the expression and purification of recombinant TNF-α. The molecular weight of the purified protein was approximately 17.5 kDa, as evidenced in Figure 1. A. To validate the specific binding affinity of the antibody with the TNF-α recombinant protein, Western blot analysis was performed (Figure 1B), providing additional evidence for the existence of TNF-α and its successful expression and purification. In conclusion, the results depicted in Figure 1 (A and B) demonstrate the effective synthesis of recombinant TNF-α protein, with the expected molecular weight of approximately 17.5 kDa.
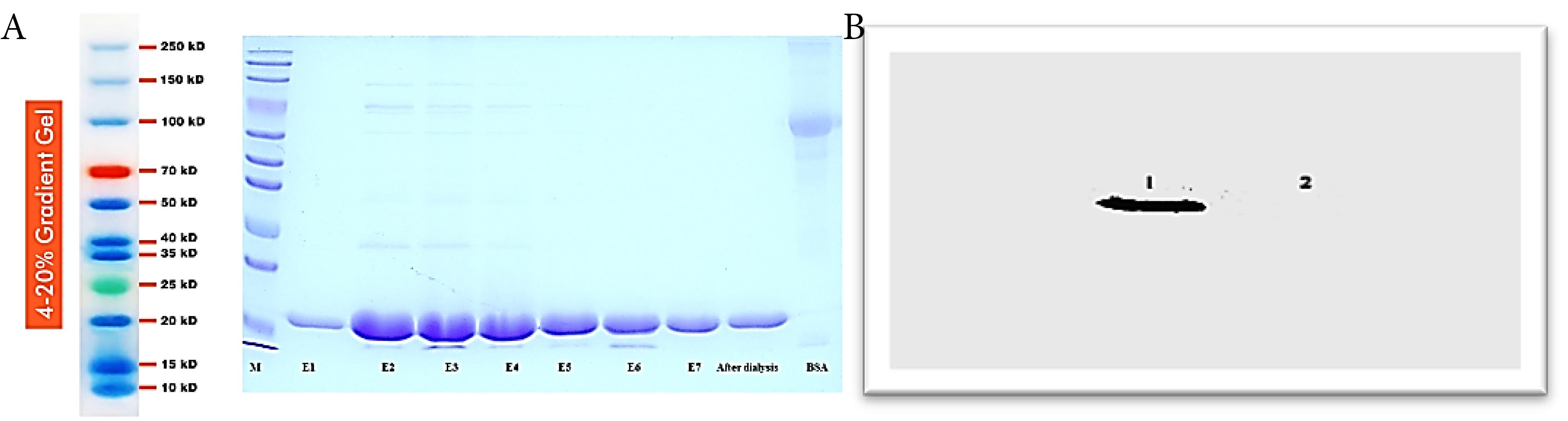
Figure 1.
(A) Illustration of the expression of 18 kD TNF-α through the action of antibodies, facilitated by the presence of histidine. Lane M represents the molecular weight marker. Lanes E1 to E7 illustrate the samples collected during column washing. The subsequent lane displays the protein following dialysis, while the final lane depicts BSA, used as a control in the experiment. (B) Illustration of the presence of TNF-α Protein in number 1 with a molecular weight of 17.5 kD. The absence of this protein in number 2 can be attributed to its lack of histidine
.
(A) Illustration of the expression of 18 kD TNF-α through the action of antibodies, facilitated by the presence of histidine. Lane M represents the molecular weight marker. Lanes E1 to E7 illustrate the samples collected during column washing. The subsequent lane displays the protein following dialysis, while the final lane depicts BSA, used as a control in the experiment. (B) Illustration of the presence of TNF-α Protein in number 1 with a molecular weight of 17.5 kD. The absence of this protein in number 2 can be attributed to its lack of histidine
The Effect of TNF-α on the Viability of MCF-7 and MDA-MB-468 Cells
The dose-dependent impact of TNF-α on cellular viability was determined, revealing different results at distinct concentrations. The results of the MTT assay indicated that MDA-MB-468 cells exhibited increased sensitivity and responsiveness to TNF-α when exposed to different concentrations of it. In contrast, the MCF-7 cells demonstrated relatively diminished sensitivity to the tested concentrations of TNF-α. The presence of an oscillatory pattern in both cell groups indicates a multifaceted and ever-changing response to TNF-α treatment, wherein the impact on cell viability may differ based on the concentration as depicted in Figure 2.
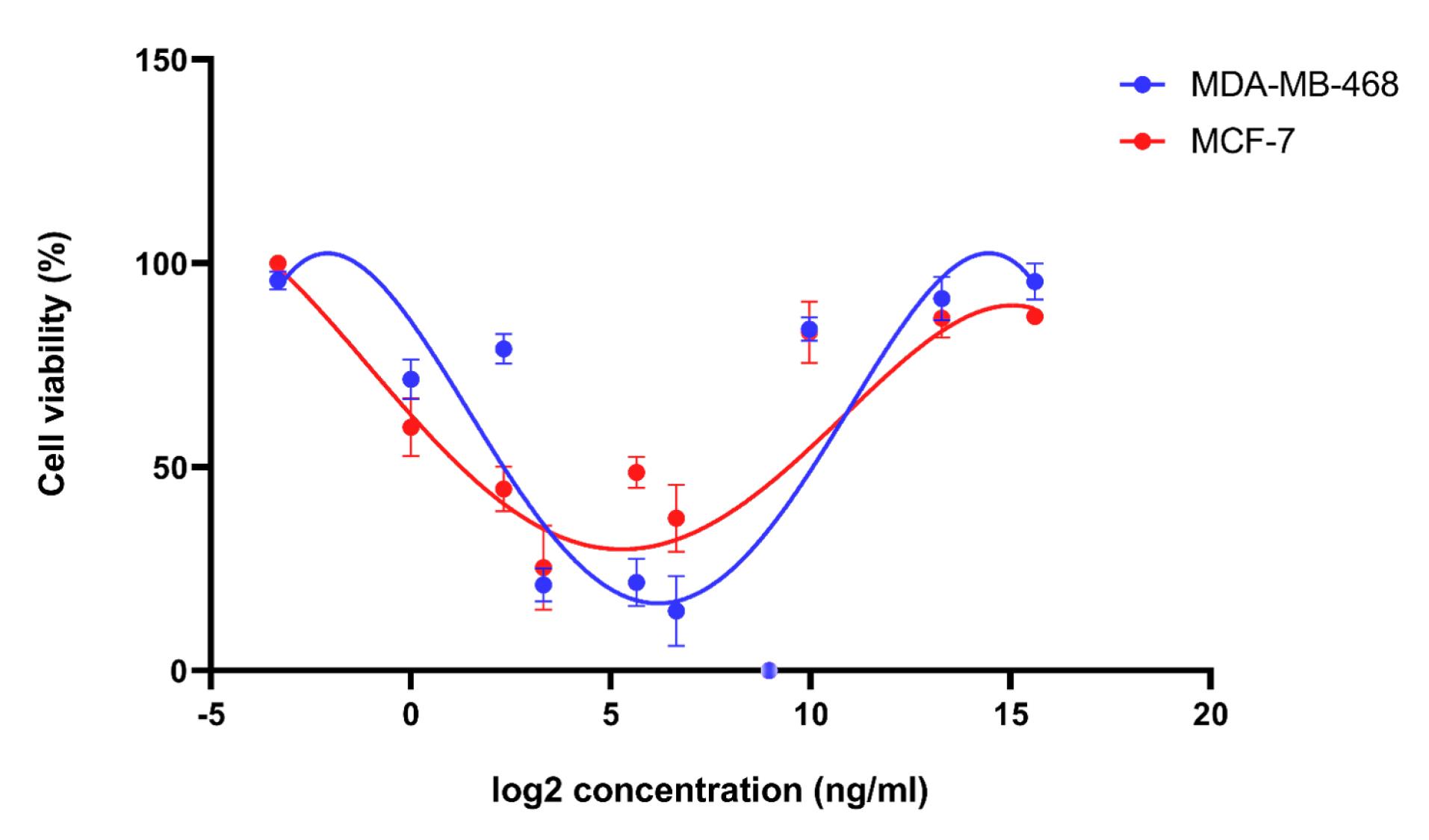
Figure 2.
The Cell Viability Curves of Recombinant TNF-α in MCF-7 Cells and MDA-MB-468 Cells, Exhibiting a Dose-Dependent Interaction. The results indicate that MDA-MB-468 cells display a higher degree of sensitivity when treated with recombinant TNF-α
.
The Cell Viability Curves of Recombinant TNF-α in MCF-7 Cells and MDA-MB-468 Cells, Exhibiting a Dose-Dependent Interaction. The results indicate that MDA-MB-468 cells display a higher degree of sensitivity when treated with recombinant TNF-α
Expression of Caspase-3 in MCF-7 and MDA-MB-468 Cells Treated With Recombinant TNF-α
A comparison was made between caspase-3 expression levels in MCF-7 and MDA-MB-468 cells following exposure to varying concentrations of recombinant TNF-α (50, 500, 1000, and 10000 ng/mL). The qRT-PCR assay yielded intriguing findings. Specifically, in MCF-7 cell lines, the administration of TNF-α at 50 ng/mL led to a significant decrease in caspase-3 expression (**P < 0.01) compared to the control group. In contrast, MDA-MB-468 cells showed considerable upregulation of caspase-3 expression when exposed to the same concentration of TNF-α (*P < 0.05) relative to the control group. Upon increasing the dose to 500 ng/mL, both cell lines displayed a more pronounced reduction in caspase-3 expression, suggesting a potential dose-dependent impact on the proliferation of MCF-7 (****P < 0.0001) and MDA-MB-468 (*P < 0.05) cells. Furthermore, treatment with 1000 ng/mL of TNF-α resulted in considerable reduction of caspase-3 expression in both cell lines, with MDA-MB-468 cells showing a more substantial decrease (****P < 0.0001) compared to MCF-7 cells (*P < 0.05). Finally, the highest concentration of TNF-α (10,000 ng/mL) exhibited the most significant reduction in caspase-3 expression among the doses studied in MCF-7 cells (****P < 0.0001). However, this dose did not yield a significant effect in MDA-MB-468 cells (Figures 3A and 3B).
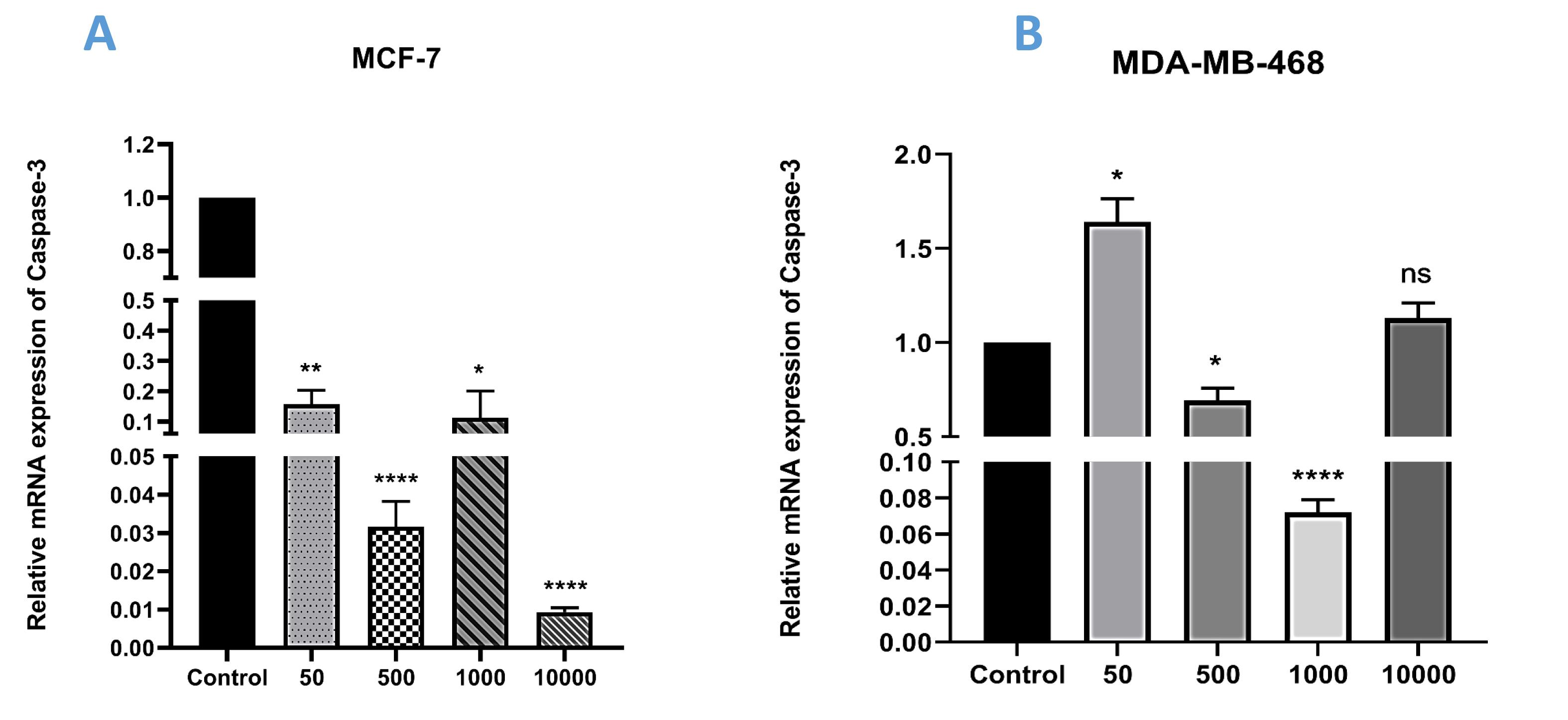
Figure 3.
Relative Expression of Caspase-3 in (A) MCF-7 and (B) MDA-MB-468 Cells Following Treatment With Four Different Doses of Recombinant TNF-α (*P < 0.05, ** P < 0.01, *** P < 0.001, and **** P < 0.0001)
.
Relative Expression of Caspase-3 in (A) MCF-7 and (B) MDA-MB-468 Cells Following Treatment With Four Different Doses of Recombinant TNF-α (*P < 0.05, ** P < 0.01, *** P < 0.001, and **** P < 0.0001)
Expression of IL-1 in MCF-7 and MDA-MB-468 Cells Treated With Recombinant TNF-α
This research investigated the impact of varying doses of TNF-α on the expression levels of IL-1, an inflammatory factor, using qRT-PCR. As shown in Figure 4, in MCF-7 cells, the expression of IL-1 significantly decreased at a dose of 50 ng/mL of TNF-α compared to the control group (P < 0.05). Conversely, this decrease was not statistically significant in MDA-MB-468 cells. However, both cell lines exhibited a considerable increase in the expression levels of IL-1 when exposed to a concentration of 500 ng/mL of TNF-α (P < 0.01). The subsequent group was administered a greater dose of TNF-α (1000 ng/mL), and analysis of the findings indicated a significant decrease in the expression of IL-1 in both cell lines when compared to the dose of 500 ng/mL, as well as the control group. However, the observed reduction was statistically more significant in the MDA-MB-468 cell line (****P < 0.0001) compared to the MCF-7 cell line (*P < 0.05), though lower P-values do not necessarily imply a greater impact. Finally, the examination of the outcomes related to the administration of the strong dose of recombinant TNF-α (10000 ng/mL) exhibited the most significant reduction in expression levels when comparing the treatment groups and the control group in the MCF-7 cell line (P < 0.01). However, the findings exhibited a contrasting pattern in the MDA-MB-468 cell line, indicating a notable rise when compared to the preceding group, especially at the concentration of 1000 ng/mL (****P < 0.0001). Our findings demonstrated that TNF-α has diverse effects at various doses.
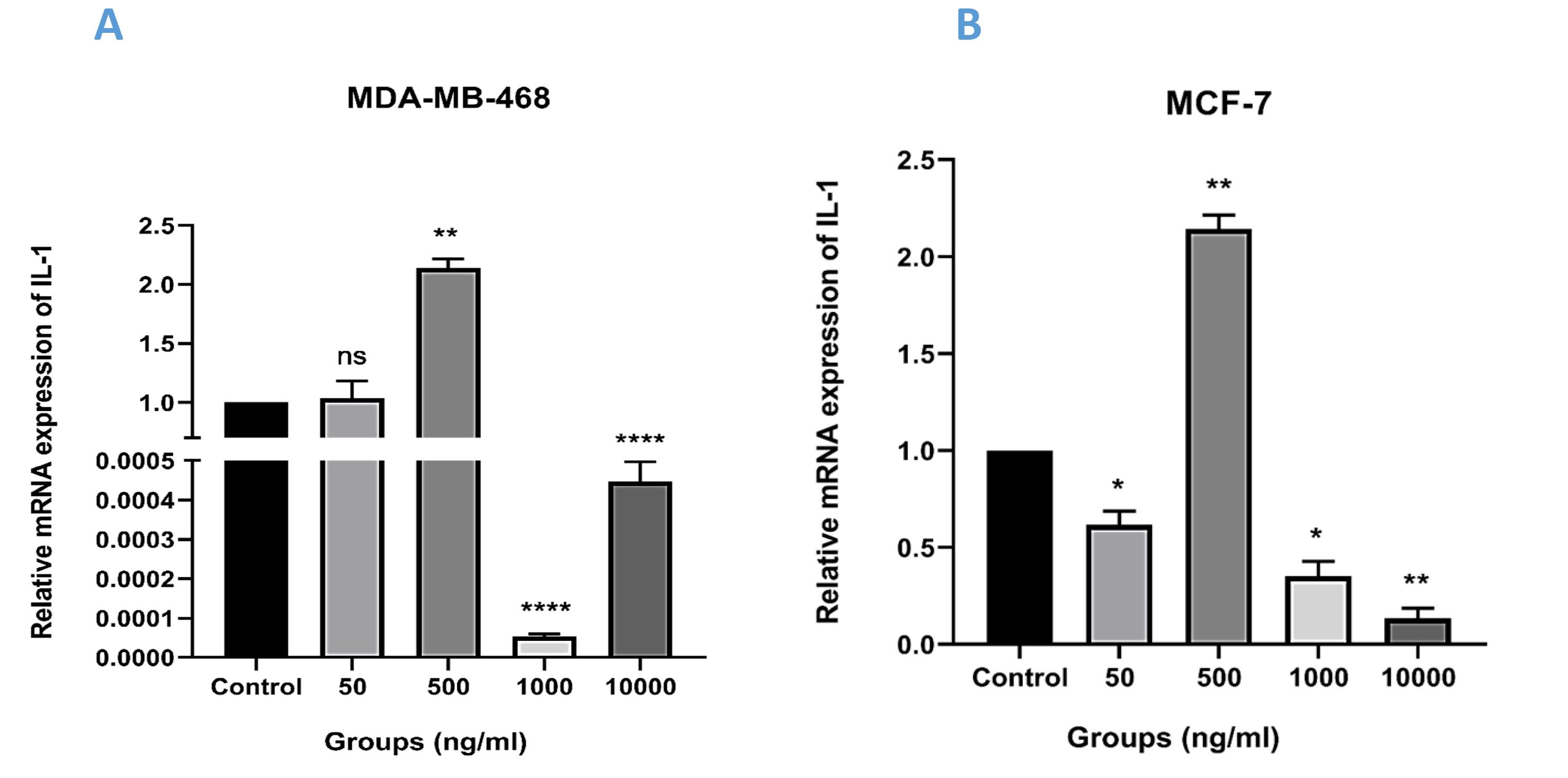
Figure 4.
Relative Expression of IL-1 in (A) MCF-7 and (B) MDA-MB-468 Cell Lines Following Treatment With Recombinant TNF-α at Four Different Concentrations (*P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001)
.
Relative Expression of IL-1 in (A) MCF-7 and (B) MDA-MB-468 Cell Lines Following Treatment With Recombinant TNF-α at Four Different Concentrations (*P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001)
Discussion
Breast cancer is a highly common condition affecting women, and the TME is the principal driver of genetic and epigenetic alterations that lead to its formation.2 Regarding breast cancer, the impact of certain mechanisms, especially the facilitation of inflammatory processes, holds significant importance in triggering both the onset and progression of the disease.24 TNF-α serves as a vital pro-inflammatory cytokine with a prominent role in regulating apoptosis and inflammation in the biological system.25 TNF-α has been identified as a pharmacological agent with anti-tumor properties, as it triggers the apoptosis of tumor cells. Nevertheless, various tumor forms, including breast cancer, have shown their complex character where the cancer facilitates cell survival by developing a connection between inflammation and the development of cancer.26 The research findings have unveiled that the presence of TNF-α in the MCF7 cell line can trigger apoptosis by activating poly ADP ribose polymerase (PARP) and caspase-dependent mechanisms. Furthermore, TNF-α could mediate the buildup of anti-apoptotic compounds like BCL-2 via a mechanism controlled by nuclear factor kappa-beta (NF-κB) and unrelated to p53.27-29 The primary objective of this investigation was to evaluate the effect of varying concentrations of recombinant TNF-α on apoptosis and cell survival across two different breast cancer cell types, namely MDA-MB-468 and MCF-7. Notably, the SDS PAGE analysis provided compelling evidence of successful protein expression, as manifested by the presence of a distinct band at approximately 17.5 kDa, consistent with previous research findings. This finding aligns with the expected size of the recombinant protein produced in the current investigation.30 Additionally, it has been demonstrated that the viability of MCF-7 cells remained at 100% when exposed to low concentrations of TNF-α. However, as the dose increased, a decline in cell survival was observed, aligning with the outcomes reported in this investigation at lower doses.31 The survival rate of MDA-MB-231 cells was positively correlated with the concentration of TNF-α, according to the research by Bauer et al.32 This finding aligns with the increasing concentration observed in the present study. Furthermore, as indicated in the reports, the administration of TNF-α at concentrations of 10 ng/mL or 100 ng/mL has been shown to increase the proliferation of cancer stem cells.33 Cai et al conducted a study that showcased a significant increase in cell proliferation in MDA-MB-468 and/or SK-BR3 cells when exposed to 20 ng/mL of TNF-α. This effect was mainly attributed to the activation of TNFR1. The researchers also excluded the potential occurrence of apoptosis facilitated by 20 ng/mL TNF-α.34 According to research findings, there is evidence to suggest that the increased regulation of TNF-α exerts a regulatory influence on the proliferation and development of neoplastic cells. On the other hand, the inadequate regulation of TNF-α performs an essential function in promoting the progression of tumors.35 However, the current investigation indicated that heightened TNF-α levels correlated with increased cellular proliferation. Another study revealed that the influence of TNF-α on apoptosis initiation was insignificant. The administration of TNF-α together with IFN-γ results in endothelial apoptosis.36 This report evaluates the particular impact of TNF-α, thereby providing a critical analysis of the findings from a prior study. IL-1β and TNF-α are two types of inflammatory cytokines that are frequently observed in the inflammatory environment of numerous tumors. When studying the impact of acute local administration of TNF-α on tumor cytotoxicity, it is evident that chronic and persistent involvement of TNF-α in tumors has notable protumoral effects in various types of cancers.14,18,37 Recent research investigating the roles of TNF-α and IL-1β in the context of cancer has led to the recognition of these cytokines as potential targets for cancer therapy.38-40 The study conducted by Katanov et al indicates that stromal cells in close proximity to cancer cells play a significant role in promoting the inflammatory response of the TME. The presence of inflammatory cytokines, including TNF-α and IL-1β, plays a pivotal role in establishing a synchronized inflammatory network that interconnects cancer cells and stromal cells in the TME.15 In this research, we set out to investigate the expression levels of the gene caspase-3, which is associated with apoptosis, as well as the factor IL-1, which is associated with pro-inflammation. The relative expression of caspase-3 in MCF-7 cells did not exhibit an obvious pattern. The expression of caspase-3 was observed in all experimental groups. However, a notable decrease in caspase-3 expression was observed as the dose increased. The observed upregulation of caspase-3 in MCF-7 cells provides evidence for the involvement of caspase-3 in both death receptor-mediated and mitochondrial-mediated apoptotic pathways. The significance of caspase-3 in the process of apoptosis and its association with changes in other regulators of apoptosis and resistance to chemotherapy underscores the potential for caspase-3 deficiency, which can substantially contribute to chemotherapeutic resistance.41 In a study conducted by Singh et al, it was observed that the activation of caspases-3 and 8 in MDA-MB-468 cells was not achieved solely by TNF-α. However, when TNF-α was combined with 1 mM NOHA at a concentration of 100 ng/mL, the activation of caspases 3 and 8 occurred within a relatively brief period of time.42 Previous research has demonstrated that the combination of TNF-α and miR-145 treatment in MDA-MB-231 cells resulted in the induction of TNF-α-induced apoptosis through the facilitation of the RIP1-FADD caspase-8 complex formation.43 Research has shown that the cytokines TNF-α and IFN-γ cause breast cancer cells to undergo cell death and decrease the expression of the HER2 protein, and this effect is dependent on the dose of these cytokines.44 Based on the findings of the study conducted by Soria, there is compelling evidence suggesting a connection between the chemokines CCL2 (MCP-1) and CCL5 (RANTES) and the cytokines TNF-α and IL-1β, with the advancement and dissemination of breast cancer. Compared to tumor cells taken from three different types of cancer patients, normal breast epithelial cells had much lower expression levels of CCL5, CCL2, TNF-α, and IL-1β. In tumor cells from patients with DCIS and IDC-no-relapse, remarkable relationships were noted between CCL2 and CCL5, together with TNF-α and IL-1β.45 Numerous research studies have provided evidence supporting the significant involvement of inflammation in the genesis and advancement of tumors.46
Conclusion
This study aimed to investigate the effect of different concentrations of recombinant TNF-α on the growth and death of MCF-7 and MDA-MB-468 breast cancer cell lines. Through comprehensive experimentation and analysis, our objective was to gain a deeper understanding of the complex relationship between TNF-α concentrations and cellular behavior. The results revealed that cellular cytotoxicity was notably higher at lower concentrations of TNF-α, while higher concentrations led to a decrease in cytotoxicity. Furthermore, we assessed the expression levels of caspase-3 and IL-1 genes, which are involved in apoptosis induction and pro-inflammatory responses, respectively. The expression patterns of caspase-3 and IL-1 in both cell lines did not demonstrate a clear and specific trend.
Ethics statement
Not Applicable.
Conflict of interests declaration
The authors declare no conflict of interests.
Acknowledgments
This study was supported by the Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
Author contributions
Conceptualization: Behzad Baradaran, Sara Tirabadi.
Data curation: Mohammad Reza Nahai.
Formal analysis: Nazila Alizadeh.
Funding acquisition: Behzad Baradaran.
Investigation: Sara Tirabadi.
Methodology: Mahya Ahmadpour Youshanlui.
Project administration: Behzad Baradaran.
Resources: Mohammad Nahai.
Software: Nazila Alizadeh.
Supervision: Behzad Baradaran.
Validation: Mohammad Nahai.
Visualization: Mahya Ahmadpour Youshanlui.
Writing–original draft: Sara Tirabadi.
Writing–review & editing: Mohammad Nahai, Behzad Baradaran.
References
- Tran BX, Vo T, Dang AK, Nguyen QN, Nguyen CT, Hoang CL. Knowledge towards cervical and breast cancers among industrial workers: results from a multisite study in Northern Vietnam. Int J Environ Res Public Health 2019; 16(21):4301. doi: 10.3390/ijerph16214301 [Crossref] [ Google Scholar]
- Safaei S, Amini M, Najjary S, Mokhtarzadeh A, Bolandi N, Saeedi H. miR-200c increases the sensitivity of breast cancer cells to Doxorubicin through downregulating MDR1 gene. Exp Mol Pathol 2022; 125:104753. doi: 10.1016/j.yexmp.2022.104753 [Crossref] [ Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68(6):394-424. doi: 10.3322/caac.21492 [Crossref] [ Google Scholar]
- Ribelles N, Perez-Villa L, Jerez JM, Pajares B, Vicioso L, Jimenez B. Pattern of recurrence of early breast cancer is different according to intrinsic subtype and proliferation index. Breast Cancer Res 2013; 15(5):R98. doi: 10.1186/bcr3559 [Crossref] [ Google Scholar]
- Alizadeh A, Hamzeh-Mivehroud M, Farajzadeh M, Moosavi-Movahedi A, Dastmalchi S. A simple and rapid method for expression and purification of functional TNF-α using GST fusion system. Curr Pharm Biotechnol 2015; 16(8):707-15. doi: 10.2174/138920101608150603152549 [Crossref] [ Google Scholar]
- Eslamkhah S, Alizadeh N, Safaei S, Mokhtarzadeh A, Amini M, Baghbanzadeh A. Micro RNA-34a sensitizes MCF-7 breast cancer cells to carboplatin through the apoptosis induction. Gene Rep 2021; 25:101361. doi: 10.1016/j.genrep.2021.101361 [Crossref] [ Google Scholar]
- Derakhshani A, Rostami Z, Safarpour H, Abdoli Shadbad M, Nourbakhsh NS, Argentiero A. From oncogenic signaling pathways to single-cell sequencing of immune cells: changing the landscape of cancer immunotherapy. Molecules 2021; 26(8):2278. doi: 10.3390/molecules26082278 [Crossref] [ Google Scholar]
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012; 21(3):309-22. doi: 10.1016/j.ccr.2012.02.022 [Crossref] [ Google Scholar]
- Nilendu P, Sarode SC, Jahagirdar D, Tandon I, Patil S, Sarode GS. Mutual concessions and compromises between stromal cells and cancer cells: driving tumor development and drug resistance. Cell Oncol (Dordr) 2018; 41(4):353-67. doi: 10.1007/s13402-018-0388-2 [Crossref] [ Google Scholar]
- Alizadeh N, Mosaferi E, Farzadi L, Majidi J, Monfaredan A, Yousefi B. Frequency of null allele of Human Leukocyte Antigen-G (HLA-G) locus in subjects to recurrent miscarriage. Int J Reprod Biomed 2016; 14(7):459-64. [ Google Scholar]
- Ghoneim K, Amer M, Al-Daly A, Mohammad A, Khadrawy F, Mahmoud M. Transaminase perturbation in certain tissues of Schistocerca gregaria (Forskal)(Orthoptera: Acrididae) by Punica granatum Linn(Lythraceae) extracts. International Journal of Current Research and Academic Review 2014; 2(6):18-34. [ Google Scholar]
- Ji H, Cao R, Yang Y, Zhang Y, Iwamoto H, Lim S. TNFR1 mediates TNF-α-induced tumour lymphangiogenesis and metastasis by modulating VEGF-C-VEGFR3 signalling. Nat Commun 2014; 5:4944. doi: 10.1038/ncomms5944 [Crossref] [ Google Scholar]
- Granger GA, Shacks SJ, Williams TW, Kolb WP. Lymphocyte in vitro cytotoxicity: specific release of lymphotoxin-like materials from tuberculin-sensitive lymphoid cells. Nature 1969; 221(5186):1155-7. doi: 10.1038/2211155a0 [Crossref] [ Google Scholar]
- Ben-Baruch A. Host microenvironment in breast cancer development: inflammatory cells, cytokines and chemokines in breast cancer progression: reciprocal tumor-microenvironment interactions. Breast Cancer Res 2003; 5(1):31-6. doi: 10.1186/bcr554 [Crossref] [ Google Scholar]
- Katanov C, Lerrer S, Liubomirski Y, Leider-Trejo L, Meshel T, Bar J. Regulation of the inflammatory profile of stromal cells in human breast cancer: prominent roles for TNF-α and the NF-κB pathway. Stem Cell Res Ther 2015; 6(1):87. doi: 10.1186/s13287-015-0080-7 [Crossref] [ Google Scholar]
- Martínez-Reza I, Díaz L, García-Becerra R. Preclinical and clinical aspects of TNF-α and its receptors TNFR1 and TNFR2 in breast cancer. J Biomed Sci 2017; 24(1):90. doi: 10.1186/s12929-017-0398-9 [Crossref] [ Google Scholar]
- Sugarman BJ, Aggarwal BB, Hass PE, Figari IS, Palladino MA Jr, Shepard HM. Recombinant human tumor necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science 1985; 230(4728):943-5. doi: 10.1126/science.3933111 [Crossref] [ Google Scholar]
- Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer 2009; 9(5):361-71. doi: 10.1038/nrc2628 [Crossref] [ Google Scholar]
- Jang DI, Lee AH, Shin HY, Song HR, Park JH, Kang TB. The role of tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. Int J Mol Sci 2021; 22(5):2719. doi: 10.3390/ijms22052719 [Crossref] [ Google Scholar]
- Ren G, Ke N, Berkmen M. Use of the SHuffle strains in production of proteins. Curr Protoc Protein Sci 2016; 85(1):5-26. doi: 10.1002/cpps.11 [Crossref] [ Google Scholar]
- Fathi-Roudsari M, Akhavian-Tehrani A, Maghsoudi N. Comparison of three Escherichia coli strains in recombinant production of reteplase. Avicenna J Med Biotechnol 2016; 8(1):16-22. [ Google Scholar]
- Ke H, Wang X, Zhou Z, Ai W, Wu Z, Zhang Y. Effect of weimaining on apoptosis and caspase-3 expression in a breast cancer mouse model. J Ethnopharmacol 2021; 264:113363. doi: 10.1016/j.jep.2020.113363 [Crossref] [ Google Scholar]
- Mantovani A, Barajon I, Garlanda C. IL-1 and IL-1 regulatory pathways in cancer progression and therapy. Immunol Rev 2018; 281(1):57-61. doi: 10.1111/imr.12614 [Crossref] [ Google Scholar]
- Ghahremani Dehbokri S, Alizadeh N, Isazadeh A, Baghbanzadeh A, Abbaspour-Ravasjani S, Hajiasgharzadeh K. CTLA-4: as an immunosuppressive immune checkpoint in breast cancer. Curr Mol Med 2023; 23(6):521-6. doi: 10.2174/1566524022666220610094716 [Crossref] [ Google Scholar]
- Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev 2006; 25(3):409-16. doi: 10.1007/s10555-006-9005-3 [Crossref] [ Google Scholar]
- Cruceriu D, Baldasici O, Balacescu O, Berindan-Neagoe I. The dual role of tumor necrosis factor-alpha (TNF-α) in breast cancer: molecular insights and therapeutic approaches. Cell Oncol (Dordr) 2020; 43(1):1-18. doi: 10.1007/s13402-019-00489-1 [Crossref] [ Google Scholar]
- Donato NJ, Klostergaard J. Distinct stress and cell destruction pathways are engaged by TNF and ceramide during apoptosis of MCF-7 cells. Exp Cell Res 2004; 294(2):523-33. doi: 10.1016/j.yexcr.2003.11.021 [Crossref] [ Google Scholar]
- Wang Y, Wang X, Zhao H, Liang B, Du Q. Clusterin confers resistance to TNF-alpha-induced apoptosis in breast cancer cells through NF-kappaB activation and Bcl-2 overexpression. J Chemother 2012; 24(6):348-57. doi: 10.1179/1973947812y.0000000049 [Crossref] [ Google Scholar]
- Ahangar NK, Khalaj-Kondori M, Alizadeh N, Mokhtarzadeh A, Baghbanzadeh A, Abdoli Shadbad M. Silencing tumor-intrinsic HHLA2 potentiates the anti-tumoral effect of paclitaxel on MG63 cells: another side of immune checkpoint. Gene 2023; 855:147086. doi: 10.1016/j.gene.2022.147086 [Crossref] [ Google Scholar]
- Zwyea S, Abdulkareem RA. Cloning, expression and bioactivity of human tumor necrosis factor alpha. Syst Rev Pharm 2020; 11(6):613-21. doi: 10.31838/srp.2020.6.91 [Crossref] [ Google Scholar]
- Martínez-Reza I, Díaz L, Barrera D, Segovia-Mendoza M, Pedraza-Sánchez S, Soca-Chafre G. Calcitriol inhibits the proliferation of triple-negative breast cancer cells through a mechanism involving the proinflammatory cytokines IL-1β and TNF-α. J Immunol Res 2019; 2019:6384278. doi: 10.1155/2019/6384278 [Crossref] [ Google Scholar]
- Bauer D, Mazzio E, Hilliard A, Oriaku ET, Soliman KFA. Effect of apigenin on whole transcriptome profile of TNFα-activated MDA-MB-468 triple negative breast cancer cells. Oncol Lett 2020; 19(3):2123-32. doi: 10.3892/ol.2020.11327 [Crossref] [ Google Scholar]
- Ji H, Cao R, Yang Y, Zhang Y, Iwamoto H, Lim S. TNFR1 mediates TNF-α-induced tumour lymphangiogenesis and metastasis by modulating VEGF-C-VEGFR3 signalling. Nat Commun 2014; 5:4944. doi: 10.1038/ncomms5944 [Crossref] [ Google Scholar]
- Cai X, Cao C, Li J, Chen F, Zhang S, Liu B. Inflammatory factor TNF-α promotes the growth of breast cancer via the positive feedback loop of TNFR1/NF-κB (and/or p38)/p-STAT3/HBXIP/TNFR1. Oncotarget 2017; 8(35):58338-52. doi: 10.18632/oncotarget.16873 [Crossref] [ Google Scholar]
- Fajardo LF, Kwan HH, Kowalski J, Prionas SD, Allison AC. Dual role of tumor necrosis factor-alpha in angiogenesis. Am J Pathol 1992; 140(3):539-44. [ Google Scholar]
- Rüegg C, Yilmaz A, Bieler G, Bamat J, Chaubert P, Lejeune FJ. Evidence for the involvement of endothelial cell integrin alphaVbeta3 in the disruption of the tumor vasculature induced by TNF and IFN-gamma. Nat Med 1998; 4(4):408-14. doi: 10.1038/nm0498-408 [Crossref] [ Google Scholar]
- Bertazza L, Mocellin S. The dual role of tumor necrosis factor (TNF) in cancer biology. Curr Med Chem 2010; 17(29):3337-52. doi: 10.2174/092986710793176339 [Crossref] [ Google Scholar]
- Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res 2006; 66(2):605-12. doi: 10.1158/0008-5472.can-05-4005 [Crossref] [ Google Scholar]
- Soria G, Ofri-Shahak M, Haas I, Yaal-Hahoshen N, Leider-Trejo L, Leibovich-Rivkin T. Inflammatory mediators in breast cancer: coordinated expression of TNFα & IL-1β with CCL2 & CCL5 and effects on epithelial-to-mesenchymal transition. BMC Cancer 2011; 11:130. doi: 10.1186/1471-2407-11-130 [Crossref] [ Google Scholar]
- Balkwill F, Mantovani A. Cancer and inflammation: implications for pharmacology and therapeutics. Clin Pharmacol Ther 2010; 87(4):401-6. doi: 10.1038/clpt.2009.312 [Crossref] [ Google Scholar]
- Mesner PW Jr, Budihardjo II, Kaufmann SH. Chemotherapy-induced apoptosis. Adv Pharmacol 1997; 41:461-99. doi: 10.1016/s1054-3589(08)61069-8 [Crossref] [ Google Scholar]
- Singh R, Pervin S, Chaudhuri G. Caspase-8-mediated BID cleavage and release of mitochondrial cytochrome c during Nomega-hydroxy-L-arginine-induced apoptosis in MDA-MB-468 cells Antagonistic effects of L-ornithine. J Biol Chem 2002; 277(40):37630-6. doi: 10.1074/jbc.M203648200 [Crossref] [ Google Scholar]
- Zheng M, Wu Z, Wu A, Huang Z, He N, Xie X. MiR-145 promotes TNF-α-induced apoptosis by facilitating the formation of RIP1-FADDcaspase-8 complex in triple-negative breast cancer. Tumour Biol 2016; 37(7):8599-607. doi: 10.1007/s13277-015-4631-4 [Crossref] [ Google Scholar]
- Rosemblit C, Datta J, Lowenfeld L, Xu S, Basu A, Kodumudi K. Oncodriver inhibition and CD4( + ) Th1 cytokines cooperate through STAT1 activation to induce tumor senescence and apoptosis in HER2 + and triple negative breast cancer: implications for combining immune and targeted therapies. Oncotarget 2018; 9(33):23058-77. doi: 10.18632/oncotarget.25208 [Crossref] [ Google Scholar]
- Soria G, Ofri-Shahak M, Haas I, Yaal-Hahoshen N, Leider-Trejo L, Leibovich-Rivkin T. Inflammatory mediators in breast cancer: coordinated expression of TNFα & IL-1β with CCL2 & CCL5 and effects on epithelial-to-mesenchymal transition. BMC Cancer 2011; 11:130. doi: 10.1186/1471-2407-11-130 [Crossref] [ Google Scholar]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow?. Lancet 2001; 357(9255):539-45. doi: 10.1016/s0140-6736(00)04046-0 [Crossref] [ Google Scholar]