Int J Drug Res Clin. 2023;1:e19.
doi: 10.34172/ijdrc.2023.e19
Review Article
Incretin-Based Therapies and the Risk of Breast Cancer in Women with Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis
Mobina Oladghaffari 1, *  , Sama Rahnemayan 2
, Sama Rahnemayan 2  , Zahra Tohid 1
, Zahra Tohid 1  , Majid Montazer 3
, Majid Montazer 3  , Farnaz Dabiri 4
, Farnaz Dabiri 4
Author information:
1Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran
2Aging Research Institute, Tabriz University of Medical Sciences, Tabriz, Iran
3Department of Cardiovascular Surgery, Imam Reza Hospital, Tabriz University of Medical Sciences, Tabriz, Iran
4Clinical Research Development Unit of Tabriz Valiasr Hospital, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Background:
This systematic review and meta-analysis assessed the data from cohort studies that compared treatment with or without incretins and the incidence of breast cancer (BC) due to the current controversies.
Methods:
PubMed, Scopus, EMBASE, Cochrane, ProQuest, Ovid, and Google Scholar were searched for relevant studies. A secondary analysis was conducted to compare the effect of glucagon-like peptide-1 receptor agonists (GLP-1RAs) and dipeptidyl peptidase-4 inhibitors (DPP-4Is) on BC development.
Results:
The meta-analysis of four studies with 58,767 patients and 461,475 controls revealed that incretin-based therapies increase the incidence of BC insignificantly (risk ratio (RR)=1.23, 95% confidence interval (CI)=0.44–3.40; P=0.69). The DPP-4I group significantly reduced the risk of BC compared to the GLP-1RA group (RR=0.43, 95% CI=0.32–0.59; P<0.00001).
Conclusion:
This study found no evidence of a substantial increase in BC incidence in patients with type 2 diabetes taking GLP-1RAs and DPP-4Is. However, DPP-4Is were less likely than GLP-1RAs to cause BC formation.
Keywords: Incretins, Breast cancer, Meta-analysis, Type 2 diabetes mellitus
Copyright and License Information
© 2023 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Breast cancer (BC) is the most common cancer among women and the most common cause of women’s cancer-related mortality.1,2 It is expected that the global incidence of BC will approximately reach 3.2 million cases per year by 2050.3 One of the major risk factors for the development of BC is diabetes mellitus (DM). According to a study, diabetic women had a 1.2 times higher probability of developing BC than non-diabetic women.5 A meta-analysis in patients with BC revealed that preexisting diabetes is linked to poor disease-free survival and overall survival.6 Similar to type 2 diabetes (T2DM), aging and obesity increase the incidence of BC.4 The risk of BC was found to be increased in postmenopausal women with a higher body mass index.7
There has been a growing interest in determining whether antidiabetic drugs could affect cancer incidence in patients with T2DM. Antidiabetic medications may alter the risk of BC in diabetic patients. Rennert et al provided evidence of a strong reverse link between metformin usage and the risk of BC.8 However, long-term use of insulin glargine and sulfonylurea is linked to a higher BC risk in women with T2DM.9,10 Incretin-based medications, including glucagon-like peptide-1 receptor agonists (GLP-1RAs) and dipeptidyl peptidase-4 inhibitors (DPP-4Is) are anti-hyperglycemic drugs that were first approved by the Food and Drug Administration (FDA) in 2010 for the management of diabetes. GLP-1RAs mainly regulate postprandial glucose metabolism by stimulating pancreatic insulin production while decreasing glucagon secretion.11 Because of their modest efficacy in decreasing glucose and minimal risk of hypoglycemia and weight gain, these medications are routinely used in individuals with T2DM.12 These agents could also lower glucose levels by reducing gastrointestinal absorption and motility, suppressing appetite, delaying gastric emptying, and improving lipid metabolism.13,14 On the other hand, DPP-4Is suppress DPP-4, so they help to stimulate post-meal insulin production by preventing endogenous GLP-1 breakdown.15
There are conflicting results about the relationship between incretin-based drug usage and BC. According to the FDA and the European Medicines Agency, GLP-1 analogues are linked to an elevated BC risk.16 GLP-1 receptors have been found on normal mammary gland tissue in preclinical research, suggesting that GLP-1 analogues could stimulate early BC formation via fibroblast growth factor 7.17 Furthermore, the GLP-1 receptor was more generally discovered in BC tissue than in normal breast tissue, noticeably in diabetic patients.18 Some safety concerns about BC events have emerged from randomized controlled trials (RCTs) among patients with diabetes.19-21 In a real-world setting, however, a prospective cohort study found no link between the usage of liraglutide and female BC.22 Likewise, other studies reported a potentially protective role of DPP-4Is in BC after long-period consumption.23,24 In a cohort study, however, overall survival did not favor the patients with BC taking DPP-4Is.25 Therefore, it is uncertain if incretin-based therapy raises the risk of BC.
Regarding the inconsistent results and existing data raising concerns about BC incidence with incretin-based drugs, we aimed to conduct a meta-analysis of all cohort studies comparing incretin-based therapies with other antidiabetic medications among women with T2DM to assess the risk of BC. In addition, we tried to compare the BC risk using DPP-4Is and GLP-1RAs.
Methods
Search Strategy
Two authors (MO and SR) independently conducted a thorough literature search to identify all cohort studies that examined the connection between incretin-based medication and BC risk in T2DM women. The literature search was conducted on PubMed, Scopus, EMBASE, Cochrane, ProQuest, Ovid, and Google Scholar. Research keywords included “incretin”, “incretin-based therapy”, “glucagon-like peptide 1 receptor agonist”, “GLP-1 receptor agonists”, “GLP-1RAs”, “liraglutide”, “semaglutide”, “exenatide”, “lixisenatide”, “dulaglutide”, “albiglutide”, “dipeptidyl peptidase-4 inhibitor”, “DPP-4 inhibitors”, “DPP4i”, “sitagliptin”, “vildagliptin”, “saxagliptin”, “alogliptin”, “linagliptin”, “type 2 diabetes mellitus”, “T2DM”, “breast neoplasm”, and “breast cancer”. It should be noted that only English-language publications were considered.
Inclusion Criteria
The current study was carried out to assess the link between incretin-based medicines (exposure) and the development of BC in T2DM women (outcome). This meta-analysis included studies that fulfilled the following criteria: (a) cohort studies, (b) being done in patients with T2DM, (c) comparing incretin-based therapy to other glucose-lowering medicines, and (d) assessing BC incidence. Publications that compared DPP-4Is or GLP-1RAs with other active antidiabetic agents were included. Two authors (MO and SR) independently assessed the title or abstract and the full articles to see whether each article met the qualification criteria for ultimate inclusion. Moreover, consensus between the two investigators and a third reviewer (FD) was obtained to resolve conflicts.
Exclusion Criteria
The papers that met the following criteria were excluded: (a) non-cohort studies (b) case reports, (c) review articles, (d) conference abstracts, (e) animal studies, (f) articles that were not accessible after mailing for full-text request from the corresponding author for two times, (g) articles that did not provide enough data for analysis, and (h) duplicate articles.
Data Extraction and Quality Assessment
Each title and abstract were initially screened, and if they met the inclusion criteria, they were then thoroughly reviewed in accordance with the eligibility criteria. The characteristics of the included studies were captured using a data extraction form (author’s name, study year, location and period of the study, sample size, study type, baseline information of the patients, type of treatment, duration of treatment, and results for effectiveness and harm outcomes).
The Newcastle-Ottawa Scale’s (NOS’s) risk of bias tool was used for the methodological quality assessment of the included cohort studies.26 It was employed to evaluate cohort studies for quality of selection (selection of the non-exposed cohort, representativeness of the exposed cohort, and ascertainment of exposure), comparability (confounding), and outcome (assessment of outcome, length, and adequacy of follow-up of cohorts). A study might receive a maximum score of 9 points. We determined that studies getting 5 points or higher are of moderate to good quality. Further, the quality of each included study was independently appraised by two authors (MO and ZT). Disagreements between the two authors were resolved by consensus with two other investigators (FD, MM). Following the conclusion of the quality assessment, data extraction started by assessing the full text of publications.
Statistical Analysis
This meta-analysis was performed to assess the risk of developing BC in women with T2DM using incretin-based drugs compared to other anti-diabetic medications. Furthermore, a second analysis was conducted, including studies that compared the effects of DPP-4Is and GLP-1RAs on the risk of BC. Based on the number of BC incidents in each group, the risk ratio (RR) and 95% confidence intervals (CIs) were calculated. Moreover, the I2 statistic was used to assess the degree of heterogeneity among the studies, and when the heterogeneity was high, a random-effects model was used. Otherwise, studies with low statistical heterogeneity were performed using fixed-effects model.27 All reported P values were 2-sided, and P < 0.05 was considered significant, and RevMan 5.3 (Review Manager, Version 5.3; The Cochrane Collaboration, 2014) was used for statistical analysis.
Results
Study Selection
Database searches yielded a total of 985 studies, of which 647 studies remained after removing duplicate records. Then, 596 studies were excluded as they were irrelevant articles, reviews, case reports, animal models, cell line studies, conference abstracts, and non-English articles by screening titles and abstracts, leaving 51 studies for full-text evaluation. Afterward, the full texts of the remaining articles were assessed in detail to evaluate if they met the inclusion criteria. The systematic review and meta-analysis included the final four studies that met the inclusion criteria. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flowchart of the study is presented in Figure 1. Overall, 520 242 participants were included after assessing the type, title, abstract, and full text of studies searched up to December 2021.
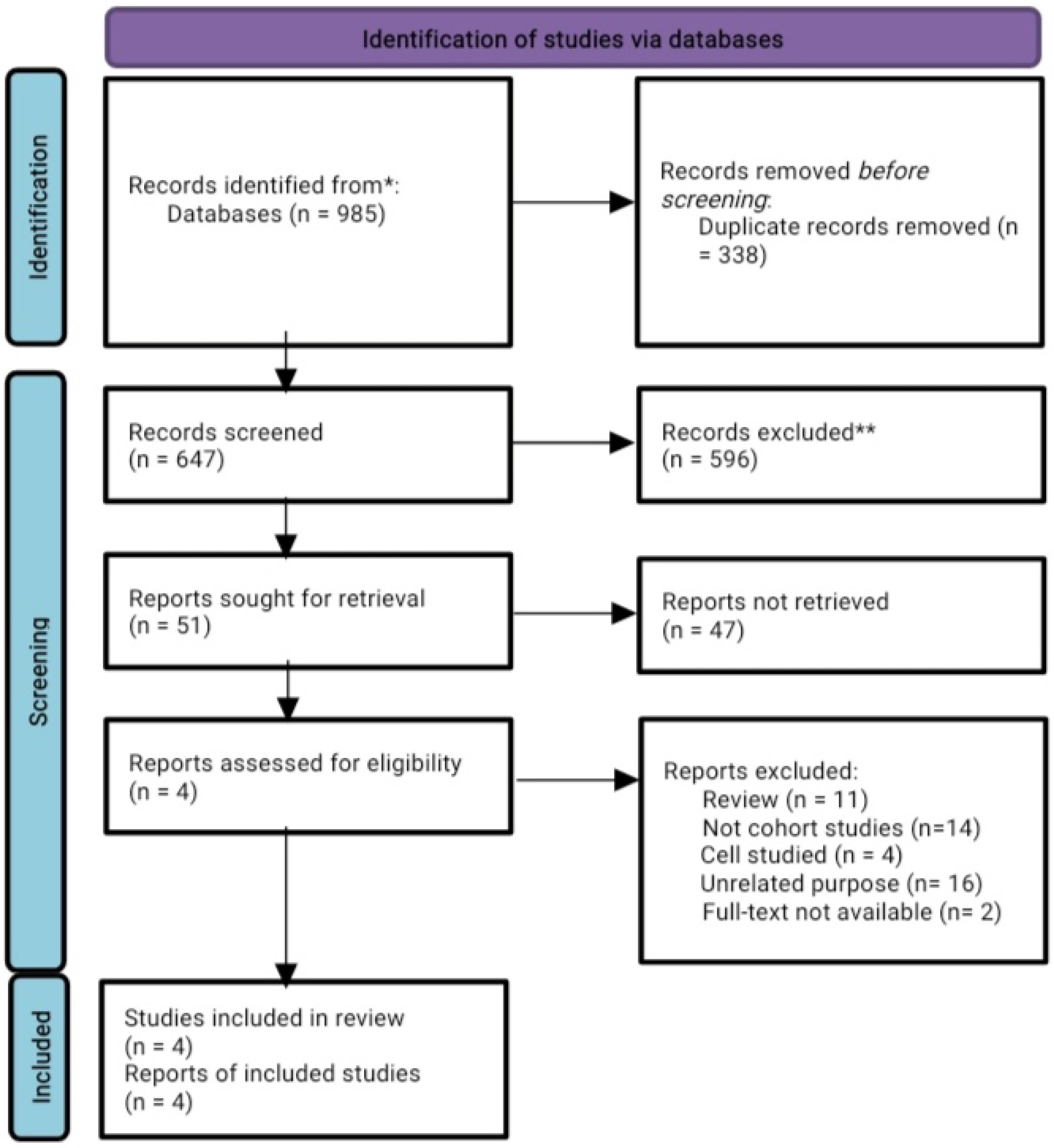
Figure 1.
Flow Diagram of the Study Selection Process
.
Flow Diagram of the Study Selection Process
Quality Assessment of Included Studies
In the systematic review, all included studies were cohort studies. Table 1 presents the study characteristics and results of the included studies. All four studies were examined by NOS and received a score of 6 points or higher, indicating that they were of moderate to good quality (Table 2).
Table 1.
Summary of the Included Studies in the Systematic Review
|
Author
|
Region
|
Year
|
Study Beginning and
Follow-up Period |
Sample
|
Instrument
|
Results
|
HR (95% CI) |
NOS
|
| Hicks et al22 |
Canada |
2016 |
1 January 2007 and 31 March 2015 to 31 March 2016 |
498 users of GLP-1RAs*, 2,422 users of DPP-4Is*, 42,064 users of other glucose-lowering drugs |
cox proportional hazards
regression models |
Among GLP-1RAs and DPP-4Is users, 31 and 68 patients had BC, respectively.
GLP-1RAs were not linked to an elevated risk of BC risk in contrast to DPP-4Is. (Incidence 4.4 v 3.4 per 1000 person-years; HR = 1.40 (95% CI = 0.91 to 2.16)). |
Crude incidence 3.5 (95%
CI = 3.3 to 3.8) per 1000 person-years |
8 |
| Tseng et al23 |
Taiwan |
2016 |
1999 and 2010 to 31
December 2011 |
32,457 users and 396,021 never-users of sitagliptin |
Cox regression models |
78 users and 2204 never-users had BC diagnoses.
Sitagliptin may lower the incidence of BC in women with T2DM, particularly one year following use. |
HR* = 0.718 (95% CI, 0.573-0.901) |
7 |
| Santella et al28 |
Canada |
2020 |
1 January 2007 to 31 January 2018 |
5,510 users of GLP-1RAs and matched 5,510 users of comparator drugs |
Cox proportional hazard models |
Among GLP-1RA users, 65 new cases of BC were diagnosed, compared with 50 patients in the comparator group. (Incidence rates in the GLP-1RAs group were 3.9 [95% CI = 3.0, 4.9] and 3.0 [95% CI = 2.2, 3.2] in the comparator group per 1,000 person-years).
According to the supplementary content, among DPP-4Is users, there were 27 BC cases.
BC diagnosis rose with GLP-1RAs weight reduction groups, especially in those who lost > 10% of their body weight. |
At least 10% weight loss (HR = 1.8, 95%
CI = 1.1, 2.8)
for > 10% weight loss (HR = 2.9,
95% CI = 1.2, 6.9) |
8 |
| Funch et al29 |
USA |
2018 |
1 February 2010 to 30 November 2014 |
17,880 users of liraglutide and 17,880 matched users of all comparator drugs |
|
Among the matched liraglutide and “all comparator”, there were 85 and 94 BC cases, respectively.
Liraglutide use did not increase the risk of BC. |
RR = 0.90 (95% CI: 0.67–1.22) |
6 |
Note. HR: Hazard ratio; NOS: Newcastle-Ottawa Scale; CI: Confidence interval; BC: Breast cancer; RR: Relative risk; GLP-1RAs: Glucagon-like peptide-1 receptor agonists; DPP-4Is: Dipeptidyl peptidase-4 inhibitors.
Table 2.
NOS Risk of Bias Tool for the Methodological Quality Assessment of the Involved Studies
|
Study ID
|
Selection
|
Comparability
|
Outcome
|
Total Score
|
| Hicks et al22 |
4 |
1 |
3 |
8 |
| Tseng23 |
4 |
1 |
2 |
7 |
| Santella et al28 |
3 |
2 |
3 |
8 |
| Funch et al29 |
3 |
1 |
2 |
6 |
Note. NOS: Newcastle-Ottawa Scale.
Meta-analysis Results
The number of BC events in each treatment arm (active medicine or placebo/non-incretin comparator drugs) was imputed for the primary analysis. Then, the RR ( ± 95% CI) was computed. The heterogeneity statistic Q was used to evaluate heterogeneity, while I2 was used to quantify it. The results demonstrated that the studies have a significant level of heterogeneity (I2 = 98%). The analysis showed that compared with antidiabetic drugs or another placebo, incretin-based therapies non-significantly increased the incidence of BC (RR = 1.23, 95% CI: 0.44–3.40; P = 0.69), as depicted in Figure 2.
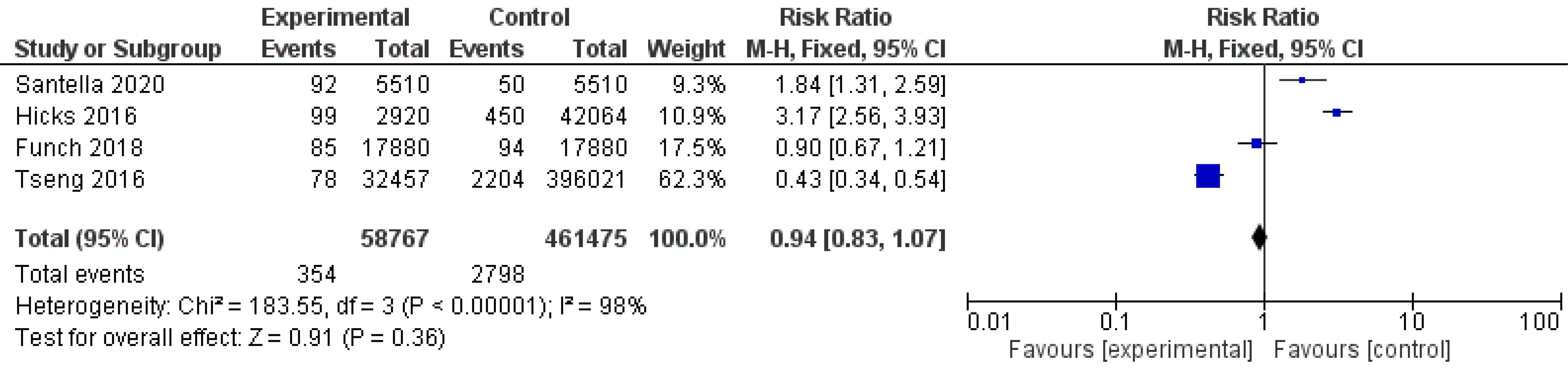
Figure 2.
Forest Plot of Comparison: BC Risk in Women with T2DM Who Were Treated with Incretin-based Medications (GLP-1RAs or DPP-4Is) Versus Other Drugs. Note. BC: Breast cancer; T2DM: Type-2 diabetes; GLP-1RAs: Glucagon-like peptide-1 receptor agonists; DPP-4Is: Dipeptidyl peptidase-4 inhibitors
.
Forest Plot of Comparison: BC Risk in Women with T2DM Who Were Treated with Incretin-based Medications (GLP-1RAs or DPP-4Is) Versus Other Drugs. Note. BC: Breast cancer; T2DM: Type-2 diabetes; GLP-1RAs: Glucagon-like peptide-1 receptor agonists; DPP-4Is: Dipeptidyl peptidase-4 inhibitors
A secondary analysis was performed to compare the effect of DPP-4Is and GLP-1RAs on BC risk. The results of the analysis indicated that BC formation is significantly lower with the use of DPP-4Is than using GLP-1RAs (RR = 0.43, 95% CI: 0.32–0.59; P < 0.00001). There was also no statistical significance in between-study heterogeneity (I2 = 0%, P = 0.79), as illustrated in Figure 3.
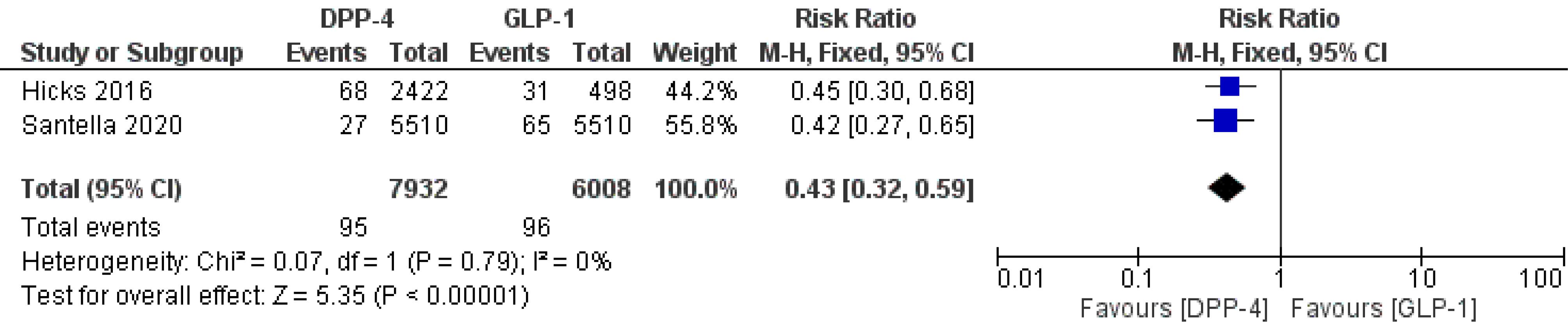
Figure 3.
Forest Plot of Comparison: Analysis of BC Risk in Women with T2DM and Comparing Treatment with GLP-1RAs and DPP-4Is Drugs. Note. BC: Breast cancer; T2DM: Type-2 diabetes; GLP-1RAs: Glucagon-like peptide-1 receptor agonists; DPP-4Is: Dipeptidyl peptidase-4 inhibitors
.
Forest Plot of Comparison: Analysis of BC Risk in Women with T2DM and Comparing Treatment with GLP-1RAs and DPP-4Is Drugs. Note. BC: Breast cancer; T2DM: Type-2 diabetes; GLP-1RAs: Glucagon-like peptide-1 receptor agonists; DPP-4Is: Dipeptidyl peptidase-4 inhibitors
Discussion
Incretin-based glucose-lowering medications (GLP-1RAs and DPP-4Is) are now routinely used to effectively treat T2DM. The advantages of their administration in diabetes are promoting weight loss (GLP-1RAs) or not affecting body weight (DPP-4Is), as well as low hypoglycemia rates.11,30 Clarifying the effect of the glucose-lowering drug on tumor biology is important because the cancer risk increases in patients with T2DM.31 Currently, a rising number of studies are focusing on the preventive function of incretin-based therapy in neurodegenerative disorders against pathophysiological processes.32 Moreover, incretin reduces diabetic microvascular complications, non-alcoholic steatohepatitis, and non-alcoholic fatty liver disease. GLP-1RAs were found to significantly reduce stroke, severe adverse cardiovascular events, and death from any cause.33-35 Despite the preventive effects of incretins, more research is needed on the cancer-related risk of these treatments.36,37
The debate about the long-term safety of incretin-based drugs is accompanied by conflicting results. A systematic review reported that once-weekly GLP-1RAs do not increase the risk of tumor occurrence.38 Additionally, DPP-4Is were not related to an increased risk of cancer at a specific site.24 However, both studies were limited to a small sample size and suggested conducting studies with a larger sample and a more extended period of follow-up to determine the long-term cancer risk. According to an observational study, in individuals with T2DM, sitagliptin may act as a preventive measure in the establishment of BC.23 However, Takigami et al demonstrated that the activation of the GLP-1 receptor might be related to the onset, progression, and treatment resistance of BC in female diabetic patients.18 Moreover, GLP-1 receptor agonists raised the risk of BC. In contrast, liraglutide could significantly lower serum tumor marker levels and apoptosis-related molecules in the breast tissues of patients with BC and diabetes.39
Likewise, in vitro studies have yielded conflicting outcomes. Increasing evidence suggests that incretin agents could have tissue-protective effects through apoptosis induction and anticancer features.40-42 Iwaya et al investigated the influence of GLP-1 against BC models, including Michigan Cancer Foundation-7 (MCF-7), MDA-MB-231 and KPL-1 cell lines, and human BC tissue. GLP-1RAs such as exendin-4 greatly decreased BC cell numbers and prevented their proliferation. Moreover, exendin-4 attenuated the growth of BC via the inhibition of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation both in vitro and in vivo.43 According to another study, depending on the cell population concentration, liraglutide could be both a stimulator and an inhibitor of MDA-MB-231 cell proliferation.44 Moreover, researchers have indicated that DPP-4 signaling can promote epidermal growth factor-induced epithelial cell transformation and mammary tumorigenesis via the induction of peptidyl-propyl cis-trans isomerase 1 expression, suggesting that sitagliptin as a DPP-4I could be useful for the treatment of breast tumors in patients with T2DM.45 On the contrary, cell lines analysis suggested that DPP-4 inhibition could accelerate BC metastasis by inducing epithelial-mesenchymal transition through C-X-C motif chemokine 12/C-X-C receptor4 (CXCL12/CXCR4)–mediated mammalian target of rapamycin activation and promote chemoresistance in BC cells.46,47 However, using DPP-4I did not enhance the risk of metastases in an observational study on diabetic patients with BC.48 A recent meta-analysis of randomized controlled trials revealed favorable outcomes for treatment with DPP-4I and BC development.49 Moreover, the weight-lowering effects of GLP-1RAs may increase BC incidence by easing the process of finding already present breast lumps, leading to early diagnosis of cancer.19,28 Furthermore, postmenopausal women who lose weight over the long term have a lower risk of BC development.50
The current study investigated the impact of incretin-based medication administration on BC incidence in women with T2DM using a meta-analysis of cohort studies. To this end, four cohort studies were included: two studies found no association between incretins and BC incidence, one indicated protective roles against BC, and one showed an increase in BC detection with incretin-based therapies.22,23,28,29 Due to the discordant results regarding the connection between incretins and BC, it was necessary to conduct a systematic review and meta-analysis of studies with longer-term follow-up.
This study indicated that in comparison to either placebo or active antidiabetic drugs, incretins are not linked to an increased BC risk, implying that there was no worrisome rise in the risk of BC during treatment with incretins. The results of the present meta-analysis are consistent with the findings of a previous systematic review and meta-analysis of RCTs conducted by Piccoli et al who analyzed the risk of BC in treatment with GLP-1RAs for diabetes and obesity.51 In another meta-analysis, the pooled estimate of observational studies on BC indicated that using DPP-4 inhibitors reduces the risk of cancer which was statistically significant (HR (95% C): 0.76 (0.60-0.96)).24 Besides, based on the limited cohort studies on incretin-based drugs, the results of the present study show no evidence of a significant rise in the incidence of BC in T2DM women taking incretin-based drugs from both the GLP-1RAs and DPP-4Is classes. The same results were found using a second analysis that classified the studies by comparing the effect of GLP-1RAs and DPP-4Is on BC risk. However, DPP-4Is were substantially associated with a lower risk of BC formation compared to GLP-1RAs. The heterogeneity test also showed a high heterogeneity among included studies, which could be a key component in the meta-analysis results. The limitation of our study lies in the limited rate of incretin use. Since the mechanisms of action of these two medication groups differ, future studies such as DPP-4I and GLP-1RA agonists should be conducted independently to improve the accuracy of the results. One of the strengths of the current review is that all of the included studies were cohort studies that, to our knowledge, have not been previously summarized in a systematic review. As the development of cancer occurs over a long period and patients participating in RCTs are healthier than general world patients, the extended duration of the studies included in the review makes it more likely to determine the risk of neoplasms with incretin-based therapies. Another strength is that we compared the effect of DPP-4Is and GLP-1RAs on the risk of BC, which has not been previously assessed.
Conclusion
In summary, findings from this study indicated that the use of DPP-4Is and GLP-1RAs is not associated with an increased BC risk. Furthermore, the DPP-4I group had a decreased risk of BC than the GLP-1RA group. Altogether, these data offer clinicians and patients further drug safety information about this potential relationship among people with T2DM.
Ethics statement
This study was approved by the Ethics Committee of Tabriz University of Medical Sciences and conducted in accordance with the Helsinki Declaration. Date: 2020-11-30, Number: IR.TBZMED.VCR.REC.1399.311.
Disclosure of funding source
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Conflict of interests declaration
The authors have no relevant financial or non-financial interests to disclose.
Acknowledgments
The authors would like to thank the staff members of the Student Research Committee, Tabriz University of Medical Sciences for their kind support (Project number: 65786).
Data Availability Statement
The corresponding author will provide the data that support the findings of this study upon reasonable request.
Consent for Publication
Not applicable.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61(2):69-90. doi: 10.3322/caac.20107 [Crossref] [ Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67(1):7-30. doi: 10.3322/caac.21387 [Crossref] [ Google Scholar]
- Hortobagyi GN, de la Garza Salazar J, Pritchard K, Amadori D, Haidinger R, Hudis CA. The global breast cancer burden: variations in epidemiology and survival. Clin Breast Cancer 2005; 6(5):391-401. doi: 10.3816/cbc.2005.n.043 [Crossref] [ Google Scholar]
- Wolf I, Sadetzki S, Catane R, Karasik A, Kaufman B. Diabetes mellitus and breast cancer. Lancet Oncol 2005; 6(2):103-11. doi: 10.1016/s1470-2045(05)01736-5 [Crossref] [ Google Scholar]
- Noto H, Goto A, Tsujimoto T, Osame K, Noda M. Latest insights into the risk of cancer in diabetes. J Diabetes Investig 2013; 4(3):225-32. doi: 10.1111/jdi.12068 [Crossref] [ Google Scholar]
- Zhao XB, Ren GS. Diabetes mellitus and prognosis in women with breast cancer: a systematic review and meta-analysis. Medicine (Baltimore) 2016; 95(49):e5602. doi: 10.1097/md.0000000000005602 [Crossref] [ Google Scholar]
- Benn M, Tybjærg-Hansen A, Smith GD, Nordestgaard BG. High body mass index and cancer risk-a Mendelian randomisation study. Eur J Epidemiol 2016; 31(9):879-92. doi: 10.1007/s10654-016-0147-5 [Crossref] [ Google Scholar]
- Rennert G, Rennert HS, Gronich N, Pinchev M, Gruber SB. Use of metformin and risk of breast and colorectal cancer. Diabetes Res Clin Pract 2020; 165:108232. doi: 10.1016/j.diabres.2020.108232 [Crossref] [ Google Scholar]
- Wu JW, Azoulay L, Majdan A, Boivin JF, Pollak M, Suissa S. Long-term use of long-acting insulin analogs and breast cancer incidence in women with type 2 diabetes. J Clin Oncol 2017; 35(32):3647-53. doi: 10.1200/jco.2017.73.4491 [Crossref] [ Google Scholar]
- Ahmadieh H, Azar ST. Type 2 diabetes mellitus, oral diabetic medications, insulin therapy, and overall breast cancer risk. ISRN Endocrinol 2013; 2013:181240. doi: 10.1155/2013/181240 [Crossref] [ Google Scholar]
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368(9548):1696-705. doi: 10.1016/s0140-6736(06)69705-5 [Crossref] [ Google Scholar]
- Ismail-Beigi F. Clinical practice Glycemic management of type 2 diabetes mellitus. N Engl J Med 2012; 366(14):1319-27. doi: 10.1056/NEJMcp1013127 [Crossref] [ Google Scholar]
- Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab 2016; 18(3):203-16. doi: 10.1111/dom.12591 [Crossref] [ Google Scholar]
- Aroda VR. A review of GLP-1 receptor agonists: evolution and advancement, through the lens of randomised controlled trials. Diabetes Obes Metab 2018; 20 Suppl 1:22-33. doi: 10.1111/dom.13162 [Crossref] [ Google Scholar]
- Drucker DJ, Sherman SI, Gorelick FS, Bergenstal RM, Sherwin RS, Buse JB. Incretin-based therapies for the treatment of type 2 diabetes: evaluation of the risks and benefits. Diabetes Care 2010; 33(2):428-33. doi: 10.2337/dc09-1499 [Crossref] [ Google Scholar]
- FDA. FDA Briefing Document - Endocrinologic and Metabolic Drugs Advisory Committee Meeting (EMDAC) for ViCTOZA (liraglutide). FDA; 2017.
- Koehler JA, Baggio LL, Yusta B, Longuet C, Rowland KJ, Cao X. GLP-1R agonists promote normal and neoplastic intestinal growth through mechanisms requiring Fgf7. Cell Metab 2015; 21(3):379-91. doi: 10.1016/j.cmet.2015.02.005 [Crossref] [ Google Scholar]
- Takigami N, Kuniyoshi S, Miki Y, Tamaki K, Kamada Y, Uehara K. Abstract P3-02-10: the possible association among breast cancer, diabetes mellitus and GLP-1 receptor. Cancer Res 2020; 80(4 Suppl):P3-02. doi: 10.1158/1538-7445.sabcs19-p3-02-10 [Crossref] [ Google Scholar]
- Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M. A randomized, controlled trial of 30 mg of liraglutide in weight management. N Engl J Med 2015; 373(1):11-22. doi: 10.1056/NEJMoa1411892 [Crossref] [ Google Scholar]
- FDA Liraglutide, 3.0 mg for Weight Management: NDA 206-321 Briefing Document. Endocrinologic and Metabolic Drug Advisory Committee, Silver Spring; 2014.
- FDA. FDA briefing document: Endocrinologic and Metabolic Drugs Advisory Committee meeting (EMDAC); 2017.
- Hicks BM, Yin H, Yu OH, Pollak MN, Platt RW, Azoulay L. Glucagon-like peptide-1 analogues and risk of breast cancer in women with type 2 diabetes: population based cohort study using the UK Clinical Practice Research Datalink. BMJ 2016; 355:i5340. doi: 10.1136/bmj.i5340 [Crossref] [ Google Scholar]
- Tseng CH. Sitagliptin may reduce breast cancer risk in women with type 2 diabetes. Clin Breast Cancer 2017; 17(3):211-8. doi: 10.1016/j.clbc.2016.11.002 [Crossref] [ Google Scholar]
- Overbeek JA, Bakker M, van der Heijden A, van Herk-Sukel MPP, Herings RMC, Nijpels G. Risk of dipeptidyl peptidase-4 (DPP-4) inhibitors on site-specific cancer: a systematic review and meta-analysis. Diabetes Metab Res Rev 2018; 34(5):e3004. doi: 10.1002/dmrr.3004 [Crossref] [ Google Scholar]
- Shah C, Hong YR, Bishnoi R, Ali A, Skelton WP 4th, Dang LH. Impact of DPP4 inhibitors in survival of patients with prostate, pancreas, and breast cancer. Front Oncol 2020; 10:405. doi: 10.3389/fonc.2020.00405 [Crossref] [ Google Scholar]
- Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better?. Mil Med Res 2020; 7(1):7. doi: 10.1186/s40779-020-00238-8 [Crossref] [ Google Scholar]
- Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 2010; 1(2):97-111. doi: 10.1002/jrsm.12 [Crossref] [ Google Scholar]
- Santella C, Yin H, Hicks BM, Yu OHY, Bouganim N, Azoulay L. Weight-lowering effects of glucagon-like peptide-1 receptor agonists and detection of breast cancer among obese women with diabetes. Epidemiology 2020; 31(4):559-66. doi: 10.1097/ede.0000000000001196 [Crossref] [ Google Scholar]
- Funch D, Mortimer K, Li L, Norman H, Major-Pedersen A, Olsen AH. Is there an association between liraglutide use and female breast cancer in a real-world setting?. Diabetes Metab Syndr Obes 2018; 11:791-806. doi: 10.2147/dmso.s171503 [Crossref] [ Google Scholar]
- Nauck MA, Vilsbøll T, Gallwitz B, Garber A, Madsbad S. Incretin-based therapies: viewpoints on the way to consensus. Diabetes Care 2009; 32(Suppl 2):S223-31. doi: 10.2337/dc09-S315 [Crossref] [ Google Scholar]
- Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA. Diabetes and cancer: a consensus report. Diabetes Care 2010; 33(7):1674-85. doi: 10.2337/dc10-0666 [Crossref] [ Google Scholar]
- Zhang D, Ma M, Liu Y. Protective effects of incretin against age-related diseases. Curr Drug Deliv 2019; 16(9):793-806. doi: 10.2174/1567201816666191010145029 [Crossref] [ Google Scholar]
- Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375(4):311-22. doi: 10.1056/NEJMoa1603827 [Crossref] [ Google Scholar]
- Wang D, Jiang L, Feng B, He N, Zhang Y, Ye H. Protective effects of glucagon-like peptide-1 on cardiac remodeling by inhibiting oxidative stress through mammalian target of rapamycin complex 1/p70 ribosomal protein S6 kinase pathway in diabetes mellitus. J Diabetes Investig 2020; 11(1):39-51. doi: 10.1111/jdi.13098 [Crossref] [ Google Scholar]
- Kimura T, Kaku K. New prospects for incretin-related drugs in the treatment of type 2 diabetes. J Diabetes Investig 2021; 12(7):1141-3. doi: 10.1111/jdi.13460 [Crossref] [ Google Scholar]
- Cristina S, Liberata S, Concetta R, Sonia F, Daniela C. Incretin-based therapies and cancer risk: a review of recent literature safety data. J Clin Diabetes Pract 2015; 1:2. doi: 10.4172/jcdp.1000102 [Crossref] [ Google Scholar]
- Karp I, Sivaswamy A, Booth C. Does the use of incretin-based medications increase the risk of cancer in patients with type-2 diabetes mellitus?. Pharmacoepidemiol Drug Saf 2019; 28(4):489-99. doi: 10.1002/pds.4746 [Crossref] [ Google Scholar]
- Guo X, Yang Q, Dong J, Liao L, Zhang W, Liu F. Tumour risk with once-weekly glucagon-like peptide-1 receptor agonists in type 2 diabetes mellitus patients: a systematic review. Clin Drug Investig 2016; 36(6):433-41. doi: 10.1007/s40261-016-0389-8 [Crossref] [ Google Scholar]
- Tang H, Wei H. Effects of liraglutide on serum tumor markers and apoptosis-related molecular levels in breast tissues of patients with breast cancer and diabetes. Anti-Tumor Pharmacy 2019; 9(4):590-5. doi: 10.3969/j.issn.2095-1264.2019.04.12 [Crossref] [ Google Scholar]
- Li XN, Bu HM, Ma XH, Lu S, Zhao S, Cui YL. Glucagon-like peptide-1 analogues inhibit proliferation and increase apoptosis of human prostate cancer cells in vitro. Exp Clin Endocrinol Diabetes 2017; 125(2):91-7. doi: 10.1055/s-0042-112368 [Crossref] [ Google Scholar]
- Amritha CA, Kumaravelu P, Chellathai DD. Evaluation of anti cancer effects of DPP-4 inhibitors in colon cancer- an invitro study. J Clin Diagn Res 2015; 9(12):FC14-6. doi: 10.7860/jcdr/2015/16015.6979 [Crossref] [ Google Scholar]
- Fidan-Yaylalı G, Dodurga Y, Seçme M, Elmas L. Antidiabetic exendin-4 activates apoptotic pathway and inhibits growth of breast cancer cells. Tumour Biol 2016; 37(2):2647-53. doi: 10.1007/s13277-015-4104-9 [Crossref] [ Google Scholar]
- Iwaya C, Nomiyama T, Komatsu S, Kawanami T, Tsutsumi Y, Hamaguchi Y. Exendin-4, a glucagonlike peptide-1 receptor agonist, attenuates breast cancer growth by inhibiting NF-κB activation. Endocrinology 2017; 158(12):4218-32. doi: 10.1210/en.2017-00461 [Crossref] [ Google Scholar]
- Shadboorestan A, Tarighi P, Koosha M, Faghihi H, Ghahremani MH, Montazeri H. Growth promotion and increased ATP-binding cassette transporters expression by liraglutide in triple negative breast cancer cell line MDA-MB-231. Drug Res (Stuttg) 2021; 71(6):307-11. doi: 10.1055/a-1345-7890 [Crossref] [ Google Scholar]
- Choi HJ, Kim JY, Lim SC, Kim G, Yun HJ, Choi HS. Dipeptidyl peptidase 4 promotes epithelial cell transformation and breast tumourigenesis via induction of PIN1 gene expression. Br J Pharmacol 2015; 172(21):5096-109. doi: 10.1111/bph.13274 [Crossref] [ Google Scholar]
- Yang F, Takagaki Y, Yoshitomi Y, Ikeda T, Li J, Kitada M. Inhibition of dipeptidyl peptidase-4 accelerates epithelial-mesenchymal transition and breast cancer metastasis via the CXCL12/CXCR4/mTOR axis. Cancer Res 2019; 79(4):735-46. doi: 10.1158/0008-5472.can-18-0620 [Crossref] [ Google Scholar]
- Li S, Fan Y, Kumagai A, Kawakita E, Kitada M, Kanasaki K. Deficiency in dipeptidyl peptidase-4 promotes chemoresistance through the CXCL12/CXCR4/mTOR/TGFβ signaling pathway in breast cancer cells. Int J Mol Sci 2020; 21(3):805. doi: 10.3390/ijms21030805 [Crossref] [ Google Scholar]
- Rathmann W, Kostev K. Association of dipeptidyl peptidase 4 inhibitors with risk of metastases in patients with type 2 diabetes and breast, prostate or digestive system cancer. J Diabetes Complications 2017; 31(4):687-92. doi: 10.1016/j.jdiacomp.2017.01.012 [Crossref] [ Google Scholar]
- Dicembrini I, Nreu B, Montereggi C, Mannucci E, Monami M. Risk of cancer in patients treated with dipeptidyl peptidase-4 inhibitors: an extensive meta-analysis of randomized controlled trials. Acta Diabetol 2020; 57(6):689-96. doi: 10.1007/s00592-020-01479-8 [Crossref] [ Google Scholar]
- Chlebowski RT, Luo J, Anderson GL, Barrington W, Reding K, Simon MS. Weight loss and breast cancer incidence in postmenopausal women. Cancer 2019; 125(2):205-12. doi: 10.1002/cncr.31687 [Crossref] [ Google Scholar]
- Piccoli GF, Mesquita LA, Stein C, Aziz M, Zoldan M, Degobi NAH. Do GLP-1 receptor agonists increase the risk of breast cancer? A systematic review and meta-analysis. J Clin Endocrinol Metab 2021; 106(3):912-21. doi: 10.1210/clinem/dgaa891 [Crossref] [ Google Scholar]