Int J Drug Res Clin. 2:e16.
doi: 10.34172/ijdrc.2024.e16
Original Article
Relation of Serum Level of Tramadol With Consumption Dose and Time
Paria Habibollahi 1, *  , Alireza Garjani 2
, Alireza Garjani 2  , Samad Shams Vahdati 3
, Samad Shams Vahdati 3 
Author information:
1Toxicology and pharmacology Department, Tabriz University of Medical Sciences, Tabriz, Iran
2Pharmacology and Toxicology Department, Tabriz University of Medical Sciences, Tabriz, Iran
3Emergency and Trauma Care Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Background:
This study aimed to find out the relationship between the serum level of tramadol and consumption dose and the time interval between the drug consumption and arrival at the emergency department (ED).
Methods:
This was a prospective cross-sectional study that included all patients who came to the ED of Sina hospital, a poisoning center, due to the consumption or abuse of tramadol within 6 months. Among these patients, those with unreliable history and history of drug use, those whose primary toxicologic screening in the ED indicated opioids and buprenorphine, and those who used tramadol chronically due to underlying disease were excluded from the study. Demographic characteristics of the patients, the type of consumed drug, the reason for use, the type of complication, and the outcome of the patient extracted from the documentation, blood samples obtained from the patients after obtaining informed consent, and the serum level of tramadol were checked and recorded using the capillary electrophoresis (CE) device.
Results:
The study investigated the serum levels of tramadol in 43 male patients presenting to the ED due to tramadol consumption or abuse. The median age was 30 years. All patients were male, and the median dose of utilized tramadol was 2000 mg. The average time from consumption to referral was 6 hours, and 14% of patients experienced seizures after taking the medication. The median serum level of tramadol was 46.12 mmol/dL. Furthermore, quadratic regression analysis revealed no significant relationship between tramadol serum levels with the dose (P=0.233) and the interval (P=0.682) between drug consumption and seeking medical attention.
Conclusion:
These findings suggested that serum levels of tramadol do not correlate with the administered dose or the time elapsed before seeking medical help.
Keywords: Serum level, Tramadol, Dose
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
The National Institute for Medical Research Development.
Please cite this article as follows: Paria Habibollahi P, Garjani A, Shams Vahdati S. Relation of Serum Level of Tramadol With Consumption Dose and Time. Int J Drug Res Clin. 2024; 2: e16. doi: 10.34172/ijdrc.2024.e16
Introduction
Tramadol is a synthetic opioid analgesic medication that is commonly prescribed for the management of moderate to severe pain.1 It works by binding to opioid receptors in the brain and spinal cord, inhibiting the transmission of pain signals.2 Tramadol is available in both immediate-release and extended-release formulations, with dosages ranging from 25 mg to 300 mg per day depending on the patient’s age, medical history, and pain severity.3-6 Although tramadol can be effective in managing pain, it is important to understand the potential risks and side effects associated with its use.
One of the most significant risks of tramadol use is its potential for addiction and abuse7. Tramadol is classified as a Schedule IV controlled substance by the US Drug Enforcement Administration (DEA), indicating a low-to-moderate risk of dependence compared to other opioids.8 However, it still has the potential for abuse and addiction, particularly in individuals with a history of substance abuse or addiction. Additionally, tramadol use can lead to a range of side effects, including dizziness and drowsiness, nausea and vomiting, constipation, headache, dry mouth, sweating, difficulty sleeping, decreased appetite, and seizures.9 It is important to note that elderly patients may be more susceptible to these side effects.10 Furthermore, tramadol use can be dangerous when combined with certain medications such as benzodiazepines, which can increase the risk of respiratory depression and other serious side effects.3
Tramadol use can also have potential long-term effects on the body, particularly with prolonged or excessive use.3 As tramadol is highly metabolized in the body, it can lead to the accumulation of toxic metabolites, increasing the risk of liver and kidney damage.10 Additionally, tramadol can cause changes in mood and behavior, including depression, anxiety, and irritability.11
The dosage and frequency of consumption are significant factors that affect tramadol serum levels12. The recommended initial dosage for adults is 25 mg per day, taken every morning, while the maximum recommended daily dose is 400 mg.13 For individuals with severe kidney problems, the doctor may prescribe 50-100 mg every 12 hours.3 The plasma elimination half-life of tramadol is around 5-6 hours, and the peak plasma levels occur about 1.5 hours after intake.14 Multiple dosing of tramadol can increase the plasma elimination half-life from six hours to seven hours.15 The hepatic metabolites of tramadol are polar, and their elimination half-life is around nine hours.16
The current study sought to find the relationship between consumption dose and the time interval between consumption and presentation in ED with serum level of tramadol. As such, any finding regarding the relationship between serum level and time of consumption or dose of consumption can help predict time or dose of consumption according to serum level.
Methods
This was a prospective cross-sectional study, jointly conducted in the Department of Pharmacology and Toxicology of the Faculty of Pharmacy, Pharmaceutical Analysis Research Center, and the ED of Sina Educational and Treatment Center affiliated with Tabriz University of Medical Sciences. Accordingly, all patients who came to the ED of Sina hospital, a poisoning center of East Azarbaijan province, due to the consumption or abuse of tramadol within 6 months (from March up to September) were included in the study. Among these patients, those who had unreliable history and history of drug use, those whose primary toxicologic screening in the ED indicated opioids and buprenorphine, and those who used tramadol chronically due to underlying disease were excluded from this study. After selecting the patients, the patient was routinely filed according to the clinical guidelines by the medical team with each patient while taking a blood sample immediately after hospitalization. Then, the checklist was completed by the researcher according to the standards, ethical obligations, and the consent of the patient or the patient’s relatives.
Demographic characteristics of the patients, the type of consumed drug, the reason for use, the type of complication, and the outcome of the patient were extracted from the documentation. Then, blood samples were obtained from the patients after obtaining informed consent, and the presence or absence of tramadol was checked and recorded using the capillary electrophoresis (CE) device. Based on Cronbach’s formula with an alpha of 5%, recent studies, and available input, the number of samples can be calculated to be 43 people.
The data were entered into the SPSS 20.0.0 statistical system and analyzed with the descriptive statistical method. The quadratic regression statistical method was used to investigate the relationship between dosage and time of administration until referral and tramadol serum level. Moreover, the statistical significance level was set at 0.05.
Results
This study comprised 43 patients. It was found that age does not follow the normal distribution based on the Kolmogorov-Smirnov statistical method (P < 0.001). The median age of the patients was 30 years, and the interquartile range (IQR) was between 27 and 45 years. All patients were male, and the median dose of the drug abused by the patients was 2000 mg with an IQR between 1800 and 2300 mg. Moreover, the average time from consumption to referral was 6 hours with an IQR of 6 to 8 hours. The history of patients revealed that six patients had seizures after taking medicine (14%). Additionally, the median serum level of tramadol was 12.46 mmol/dL, and the interquartile range was 96.45 to 26.46 (Figure 1).
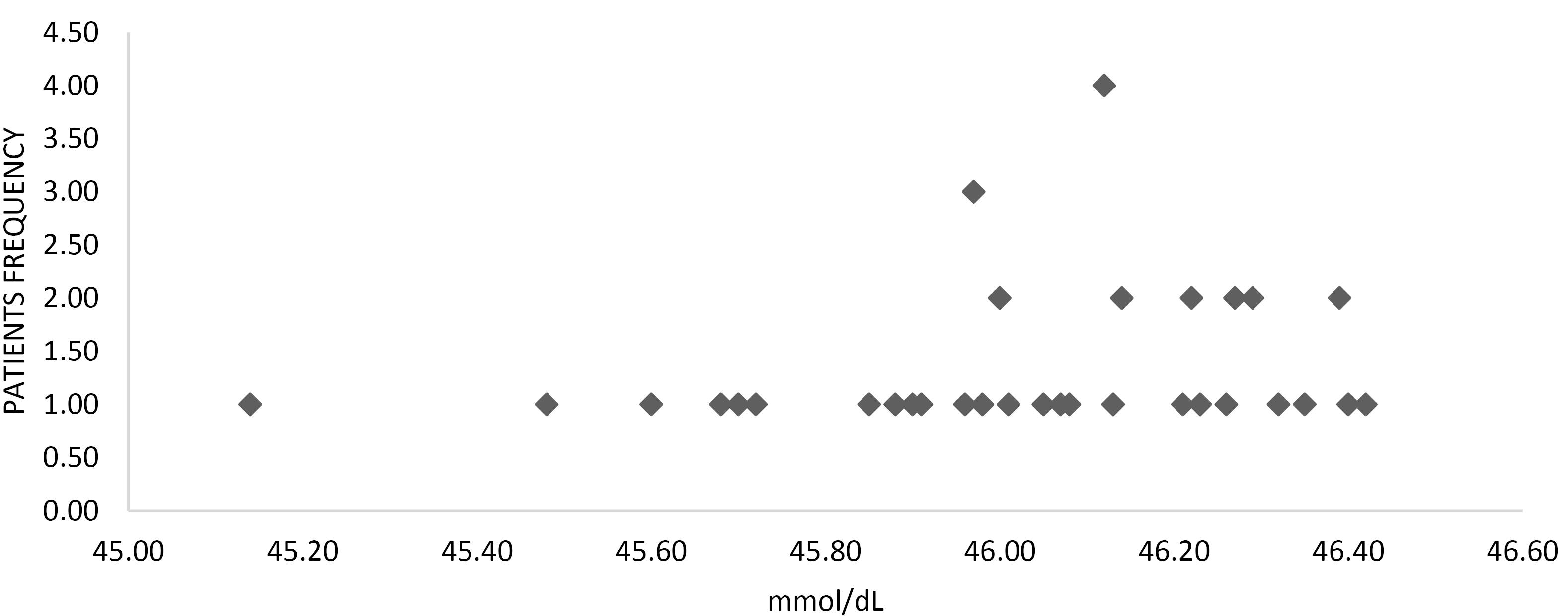
Figure 1.
The Serum Level of Patients (mmol/dL)
.
The Serum Level of Patients (mmol/dL)
Investigating the relationship between the serum level of tramadol with the dose and the interval between consumption and going to the ED using quadratic regression revealed no significant relationship between the serum level of tramadol with the dose of consumption (P = 0.233) and the time interval of ingestion to management (P = 0.682).
Discussion
The current study sought to evaluate the relationship between consumption time and dose with serum levels of tramadol. There have been no studies to predict the dose or time of tramadol consumption with the evaluation of serum level of tramadol up to now, so this study is a novel one and cannot be compared with other studies. The median age of the patients in this study was 30 years, with an age range between 27 and 45 years, and all participants were male.
The tramadol metabolism involves the formation of three primary metabolites, with each metabolite further metabolized into four pairs of enantiomers.17 This complex metabolic process can lead to variations in the serum levels of tramadol and its metabolites among different individuals. Additionally, similar to other opioid medications, tramadol poses a risk for tolerance, dependence, and abuse.15 Thus, individual differences in metabolism and elimination, as well as the potential for abuse should be considered when determining the appropriate dosage and frequency of tramadol consumption.
The median dose of tramadol used by the patients was 2000 mg, with an interquartile range between 1800 and 2300 mg. Moreover, the average time from drug consumption to referral to the ED was 6 hours, with a median ranging from 6 to 8 hours.
For acute pain, an initial dose of 100 mg is usually necessary, followed by doses of 50 or 100 mg every 4-6 hours, and the duration of treatment should be limited to five days.5,18 The maximum dosage of tramadol should not exceed 400 mg/d, and the lowest effective dosage should be used for the shortest duration consistent with the patient’s treatment goals.13,19
Monitoring tramadol serum levels is essential for safe and effective use.4,15,20 The therapeutic serum concentration of tramadol and its metabolite M1 varies significantly, with the lowest mean concentrations measured in the ( + )-group and the highest in the (-)-group4. Following a single oral dose of 100 mg of tramadol, the Cmax was found to be approximately 300μg/L with a Tmax of 1.6-1.9 hours, while metabolite M1 was found to have a Cmax of approximately 70 μg/L with a Tmax of 3.7-4.4 hours.15 The time to peak plasma concentrations for the extended-release tramadol Ultram-ER is 12 hours.20 In our study, the median serum level of tramadol was reported as 46.12 mmol/dL, with an interquartile range between 45.96 and 46.26.
Tramadol may cause serious or life-threatening breathing problems, especially during the first 24 to 72 hours of treatment and any time the dosage is increased.9 Combining tramadol with alcohol or other CNS-depressant medicines may worsen the side effects of the drug.10 Tramadol has a risk for abuse and addiction, which can lead to overdose and death.8 Before taking tramadol, patients should inform their healthcare provider of any medical conditions, including liver or kidney disease, and any medications they are currently taking.19 In our study, six individuals (14%) experienced seizures after taking the medicine.
Interestingly, when investigating the relationship between the serum level of tramadol and both the dose and the interval between drug consumption and arrival at the ED using quadratic regression, no significant relationship was found. Specifically, there was no significant correlation between the serum level of tramadol and the number of drugs taken (P = 0.233) or the time of drug administration (P= 0.682).
Limitations
Firstly, tramadol serum level monitoring may add to the overall cost of treatment, especially if frequent tests are required, potentially creating financial burdens for patients. Furthermore, the interpretation of tramadol serum levels can be challenging and may require specialized knowledge, which could lead to potential misinterpretation and inappropriate clinical decisions if not handled by experienced professionals.
Conclusion
These findings suggested that in this particular study, the serum levels of tramadol do not show a clear association with either the dose of the drug or the time elapsed between drug consumption and seeking medical attention. Further research and larger sample sizes may be warranted to validate these results and explore potential contributing factors to the observed outcomes
Ethics statement
All patients gave informed consent, and the Ethics Committee of Tabriz University of Medical Sciences approved the study (Ethics code: IR. NIMAD. REC.1396.306).
Conflict of interests declaration
None.
Acknowledgments
The authors express their Special thanks to Dr. Abolgasem Jouyban for his help and support, as well as the National Institute for Medical Research Development.
Author contributions
Conceptualization: Samad Shams Vahdati.
Data curation: Paria Habibollahi.
Formal analysis: Paria Habibollahi.
Funding acquisition: Alireza Garjani.
Investigation: Paria Habibollahi.
Methodology: Alireza Garjani.
Project administration: Alireza Garjani.
Resources: Alireza Garjani.
Software: Samad Shams Vahdati.
Supervision: Samad Shams Vahdati.
Validation: Alireza Garjani.
Visualization: Samad Shams Vahdati.
Writing–original draft: Paria Habibollahi.
Writing–review & editing: Paria Habibollahi.
References
- Santos Garcia JB, Lech O, Campos Kraychete D, Rico MA, Hernández-Castro JJ, Colimon F. The role of tramadol in pain management in Latin America: a report by the Change Pain Latin America Advisory Panel. Curr Med Res Opin 2017; 33(9):1615-21. doi: 10.1080/03007995.2017.1354821 [Crossref] [ Google Scholar]
- Miotto K, Cho AK, Khalil MA, Blanco K, Sasaki JD, Rawson R. Trends in tramadol: pharmacology, metabolism, and misuse. Anesth Analg 2017; 124(1):44-51. doi: 10.1213/ane.0000000000001683 [Crossref] [ Google Scholar]
- Beakley BD, Kaye AM, Kaye AD. Tramadol, Pharmacology, side effects, and serotonin syndrome: a review. Pain Physician 2015; 18(4):395-400. [ Google Scholar]
- Grond S, Meuser T, Uragg H, Stahlberg HJ, Lehmann KA. Serum concentrations of tramadol enantiomers during patient-controlled analgesia. Br J Clin Pharmacol 1999; 48(2):254-7. doi: 10.1046/j.1365-2125.1999.00986.x [Crossref] [ Google Scholar]
- Dyderski S, Szkutnik D, Zgrabczyńska M, Drobnik L. Bioavailability of tramadol hydrochloride from tramadol--capsules 50 mg. Acta Pol Pharm 2001; 58(5):345-9. [ Google Scholar]
- Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet 2004; 43(13):879-923. doi: 10.2165/00003088-200443130-00004 [Crossref] [ Google Scholar]
- Roussin A, Doazan-d’Ouince O, Géniaux H, Halberer C. Evaluation of abuse and dependence in addiction monitoring systems: tramadol as an example. Therapie 2015; 70(2):203-21. doi: 10.2515/therapie/2015014 [Crossref] [ Google Scholar]
- Bamigbade TA, Langford RM. The clinical use of tramadol hydrochloride. Pain Rev 1998; 5(3):155-82. [ Google Scholar]
- Habibollahi P, Garjani A, Shams Vahdati S, Sadat-Ebrahimi SR, Parnianfard N. Severe complications of tramadol overdose in Iran. Epidemiol Health 2019; 41:e2019026. doi: 10.4178/epih.e2019026 [Crossref] [ Google Scholar]
- Habibollahi P, Poureskandari M, Makouie M, Amiri H, Delice O, Rahimi A. Evaluation of neurological symptoms of acute tramadol poisoning and its relationship with laboratory findings. J Res Clin Med 2023; 11(1):7. doi: 10.34172/jrcm.2023.32201 [Crossref] [ Google Scholar]
- Kabel JS, van Puijenbroek EP. [Side effects of tramadol: 12 years of experience in the Netherlands]. Ned Tijdschr Geneeskd 2005;149(14):754-7. [Dutch].
- Ponugoti RR, Gonugunta CR. Formulation and evaluation of mouth dissolving tablets of tramadol hydrochloride. Trop J Pharm Res 2014; 13(5):669-75. doi: 10.4314/tjpr.v13i5.3 [Crossref] [ Google Scholar]
- Gendle R, Kaushik B, Verma S, Patel R, Singh SK, Namdeo KP. Formulation and evaluation of sustained release matrix tablet of tramadol HCl. Int J Chemtech Res 2010; 2(1):4-10. [ Google Scholar]
- Jick H, Derby LE, Vasilakis C, Fife D. The risk of seizures associated with tramadol. Pharmacotherapy 1998; 18(3):607-11. doi: 10.1002/j.1875-9114.1998.tb03123.x [Crossref] [ Google Scholar]
- Habibollahi P, Garjani A, Shams Vahdati S, Saadat Ebrahimi SR, Parnianfard N, Zakeri R. Characteristics of patients with tramadol use or abuse: a systematic review and meta-analysis. Eurasian J Emerg Med 2020; 19(3):127-35. doi: 10.4274/eajem.galenos.2020.71473 [Crossref] [ Google Scholar]
- Miotto K, Cho AK, Khalil MA, Blanco K, Sasaki JD, Rawson R. Trends in Tramadol: Pharmacology, Metabolism, and Misuse. Anesth Analg 2017; 124(1):44-51. doi: 10.1213/ane.0000000000001683 [Crossref] [ Google Scholar]
- Habibollahi P, Samadi A, Garjani A, Shams Vahdati S, Sargazi HR, Jouyban A. Ultrasound-assisted dispersive liquid-liquid microextraction and capillary electrophoretic determination of tramadol in human plasma. Curr Anal Chem 2021; 17(3):426-33. doi: 10.2174/1573411016666200319101416 [Crossref] [ Google Scholar]
- Guay DR. Adjunctive agents in the management of chronic pain. Pharmacotherapy 2001; 21(9):1070-81. doi: 10.1592/phco.21.13.1070.34622 [Crossref] [ Google Scholar]
- Rahmani AH, Jamshidi F, Bayat N. Tramadol poisoning and prevalence of common side effect. Int J Pharma Res Health Sci 2016; 4(5):1429-32. doi: 10.21276/ijprhs.2016.05.17 [Crossref] [ Google Scholar]
- Subedi M, Bajaj S, Kumar MS, Mayur YC. An overview of tramadol and its usage in pain management and future perspective. Biomed Pharmacother 2019; 111:443-51. doi: 10.1016/j.biopha.2018.12.085 [Crossref] [ Google Scholar]