Int J Drug Res Clin. 2:e25.
doi: 10.34172/ijdrc.2024.e25
Original Article
Effects of Soybean Consumption on Disease Severity, Quality of Life, IBD-Related Disabilities, and Mental Health in Patients with Ulcerative Colitis: A Clinical Trial
Sina Naghshi 1, 2  , Sevil Kiani 3, Helda Tutunchi 3, Omid Sadeghi 4, Leila Azadbakht 5, Seyed Ali Keshavarz 2, Amir Ali Sohrabpour 6, Manouchehr Khoshbaten 7, Ahmad Esmaillzadeh 4, 5, 8, *
, Sevil Kiani 3, Helda Tutunchi 3, Omid Sadeghi 4, Leila Azadbakht 5, Seyed Ali Keshavarz 2, Amir Ali Sohrabpour 6, Manouchehr Khoshbaten 7, Ahmad Esmaillzadeh 4, 5, 8, * 
Author information:
1Students’ Scientific Research Center, Tehran University of Medical Sciences, Tehran, Iran
2Department of Clinical Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran
3Endocrine Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
4Department of Community Nutrition, School of Nutrition and Food Science, Isfahan University of Medical Sciences, Isfahan, Iran
5Department of Community Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran
6The Liver, Pancreatic, and Biliary Disease Research Center, Digestive Disease Research Institute, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran
7Liver and Gastrointestinal Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
8Obesity and Eating Habits Research Center, Endocrinology and Metabolism Molecular -Cellular Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran
Abstract
Background:
Several dietary and non-dietary approaches have been recommended to help managing the clinical symptoms of ulcerative colitis (UC). One potential approach is the consumption of soybean due to its anti-inflammatory properties, which may aid in controlling disease severity. However, no research has been conducted on the effects of soybean consumption on disease activity in individuals with UC. To address this gap, a randomized controlled clinical trial was conducted to investigate the effect of soybean consumption on disease severity, quality of life, disability caused by the disease, and mental health in patients with mild to moderate UC.
Methods:
Thirty participants were assigned to either receive 30 g/d of soybean plus routine UC treatments (n=15) in the intervention group or only routine UC treatments (n=15) in the non-intervention group for 8 weeks.
Results:
At the end of the trial, anxiety score of the patients in the soybean group was significantly reduced compared to the control group (P=0.02). However, no significant differences were observed in depression and psychological distress scores between groups. Moreover, there were no significant differences between groups in terms of quality of life, disease severity, and inflammatory bowel disease (IBD)-related disabilities.
Conclusion:
Findings from this study provide evidence that soybean consumption significantly improves anxiety of patients with UC, while no significant changes were found in terms of quality of life, disease severity, IBD-related disabilities, depression, and psychological distress. Further studies are required to confirm these findings.
Keywords: Soybean, Quality of life, Mental health, Ulcerative colitis, Inflammation
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
The study was financially supported by the Tehran University of Medical Sciences.
Please cite this article as follows: Naghshi S, Kiani S, Tutunchi H, Sadeghi O, Azadbakht L, Keshavarz SA, et al. Effects of soybean consumption on disease severity, quality of life, IBD-related disabilities, and mental health in patients with ulcerative colitis: a clinical trial. Int J Drug Res Clin. 2024; 2: e25. doi: 10.34172/ijdrc.2024.e25
Introduction
Inflammatory bowel disease (IBD) is an autoimmune condition characterized by chronic inflammation of the digestive tract.1 It encompasses two main diseases: Crohn’s disease (CD) and ulcerative colitis (UC).2 Similar to other gastrointestinal diseases, IBD places a significant burden on the healthcare system worldwide. Estimates indicate that 10 576 000 individuals are affected by IBD worldwide. In Iran, a review study reported a prevalence of 40.67 per 100 000 people.3
Various environmental factors, including dietary intakes, can influence the symptoms and frequency of IBD flare-ups.4,5 Epidemiological studies and clinical trials have demonstrated that a high intake of fermented dairy products, probiotics, and omega-3 fatty acids can improve UC symptoms and reduce disease severity.6 Conversely, consumption of fermentable carbohydrates, spicy foods, red meat, fried foods, alcohol, and coffee has been shown to aggravate the symptoms.7-11 Additionally, some studies suggest that vitamin D deficiency and high iron intake can exacerbate UC by increasing inflammatory processes.12,13 Therefore, dietary intakes seem to play a crucial role in both preventing UC flares and managing its symptoms.
Soybean is rich in fiber, phytoestrogens, phytosterols, phospholipids, and biologically active peptides, all of which possess antioxidant, anti-inflammatory, and immune-regulating properties.14 As such, soybean could serve as a complementary treatment for UC, given that oxidant/antioxidant imbalance and inflammatory conditions significantly contribute to disease recurrence and progression. However, most studies in this area have focused on the effects of soy-derived compounds, and no research has examined the effects of whole soybean, which contains all these beneficial compounds. In an experimental study, soy protein intake in mice with UC reduced weight loss, colon shortening, splenomegaly, and colonic inflammation, leading to decreased disease activity and increased remission periods.15 Similarly, in a randomized clinical trial, the administration of Bowman-Birk inhibitor (BBI) to patients with UC reduced disease activity and increased remission periods.16 These findings underscore the need for further research to explore the effects of whole soy intake in individuals with UC.
Given the aforementioned points, we hypothesized that soybean consumption might positively impact several clinical outcomes in individuals with UC. This parallel clinical trial was designed to investigate the effect of soybean consumption on disease severity, quality of life, IBD-related disabilities, and mental health in patients with UC.
Methods
The present investigation is a randomized controlled clinical trial. Participants were recruited from Shariati and Imam Khomeini Hospitals, affiliated with Tehran University of Medical Sciences, Tehran, Iran.The study was registered on the Iranian Registry of Clinical Trials website (http://www.irct.ir) on January 24, 2020, with the code number IRCT20191113045432N1.
Participants of the current study were patients with UC, who met the following inclusion criteria: aged between 20–60 years, diagnosed with UC by a gastroenterologist using Porto diagnostic criteria based on clinical, endoscopic, radiological, histopathologic, and surgical findings, and presenting with mild to moderate disease severity. Exclusion criteria included pregnancy, lactation, smoking, alcohol consumption, intake of multivitamins, changes in the type or dosage of medications over the last three months, hospitalization in the last three months, and other pathological conditions affecting the gut such as cancer and infectious diseases. Additionally, individuals who had an infection over the past three months were not included.
Sample Size
The sample size was calculated based on disease severity.17 The minimal sample size was determined to be 13 patients per group, considering a 95% confidence interval (CI) and 80% power. The sample size was expanded to 15 patients per group to account for a possible 10% dropout rate.
Randomization
The patients were randomly allocated into either a “soybean” or “control” group in a 1:1 ratio using Random Allocation Software (RAS). Allocation was performed by a third person not involved in the study, and sequentially numbered concealed envelopes were used for allocation concealment.
Study Design and Intervention
All participants provided written informed consent and completed a comprehensive questionnaire via face-to-face interviews, which included demographic characteristics, past medical and medication history, and socioeconomic status (SES). The intervention group was instructed to consume 30 g of soybean daily in addition to their routine treatment regimens, while the non-intervention group only received routine treatment regimens. Patients in both groups also received usual nutritional recommendations for patients with IBD based on the European Society for Parenteral and Enteral Nutrition (ESPEN) guidelines.18
Outcomes
The outcomes of the present clinical trial were disease activity, quality of life, disability caused by the disease, and mental health.
Assessment of Disease Severity
Disease activity was measured at the beginning and end of the trial using the 9-point partial Mayo Clinic score developed by Sutherland et al.19,20 This score considers stool frequency, rectal bleeding, and the physician’s assessment of disease activity, with higher scores indicating greater severity.
Assessment of Disability Caused by the Disease
Disability caused by the disease was assessed using the inflammatory bowel disease disability index (IBD-DI).21,22 This questionnaire consists of 28 questions related to health, body functions, activities, and environmental factors. The answers to these questions are “yes” and “no” or based on a 5-point Likert scale (1 indicating no difficulty and 5 indicating extreme difficulty). Scores from each question were combined to obtain a total score for each domain. The final score of the questionnaire ranges from -80 (maximum disability) to 22 (no disability), with zero as the anticipated point of neutrality.
Assessment of Quality of Life
Quality of life was evaluated using the inflammatory bowel disease questionnaire-9 (IBDQ-9),23 containing nine questions about gastrointestinal disorders, and systemic, emotional, and social symptoms, with higher scores indicating a better quality of life.
Assessment of Mental Health
Given the high prevalence of psychological disorders such as depression, anxiety, and psychological distress among patients with IBD,24,25 mental health was also evaluated. The Iranian-validated version of the Hospital Anxiety and Depression Scale (HADS) was used to screen for depression and anxiety, as it is a brief and effective questionnaire for measuring the severity of anxiety and depression symptoms.26 The General Health Questionnaire (GHQ) was also employed to assess psychological distress, consisting of 28 items scored on a 4-option Likert scale.27-29
Dietary Intake and Physical Activity Assessment
Dietary intake was assessed using a three-day food recall (two non-consecutive weekdays and one day on the weekend) at baseline, middle, and end of the study. The nutrient intakes of study participants were computed using Nutritionist IV software, modified for Iranian foods. To evaluate physical activity levels, participants were asked to record their physical activity at the beginning, middle, and end of the intervention, and responses were converted to metabolic equivalent task hours per day (MET-hour/day).
Adherence
Adherence to the study intervention was evaluated using three-day dietary records. Moreover, patients were asked to return the rest of the soy packets, and weekly phone calls were made to all subjects to ensure their regular consumption.
Statistical Analysis
All analyses were conducted using an intention-to-treat (ITT) approach. The normality of variable distribution was assessed using the Shapiro-Wilk test, skewness, Q-Q diagram, and Kolmogorov-Smirnov test. Results were presented as mean (standard deviation) or frequency (percentage). Differences in categorical variables between the soybean and control groups were examined using the Chi-square test. The independent samples t-test was also used to compare the means (standard deviation) of normally distributed variables between the two groups. Within-group variations were assessed using the paired samples t-test for data with normal distribution. Additionally, the analysis of covariance (ANCOVA) was conducted to examine the effects of soybean consumption on outcome variables after controlling for potential confounding factors. Baseline values of outcome variables and age were adjusted to detect independent results. Analyses were performed using SPSS software version 18.0, and P < 0.05 was considered statistically significant.
Results
Figure 1 displays the CONSORT flowchart of the trial. A total of 26 participants completed the trial, with 2 patients in each arm lost to follow-up due to non-adherence to dietary recommendations. No side effects were reported by those who completed the trial, except for diarrhea, which was reported by some participants.Table 1 provides the baseline characteristics of the patients, which were well balanced between two groups. Table 2 illustrates the dietary intakes of participants, with a significant difference in fiber intake between the study groups (P = 0.04), indicating a higher fiber intake in the soybean group than in the control group. Other dietary intakes did not show statistically significant differences between the study groups.
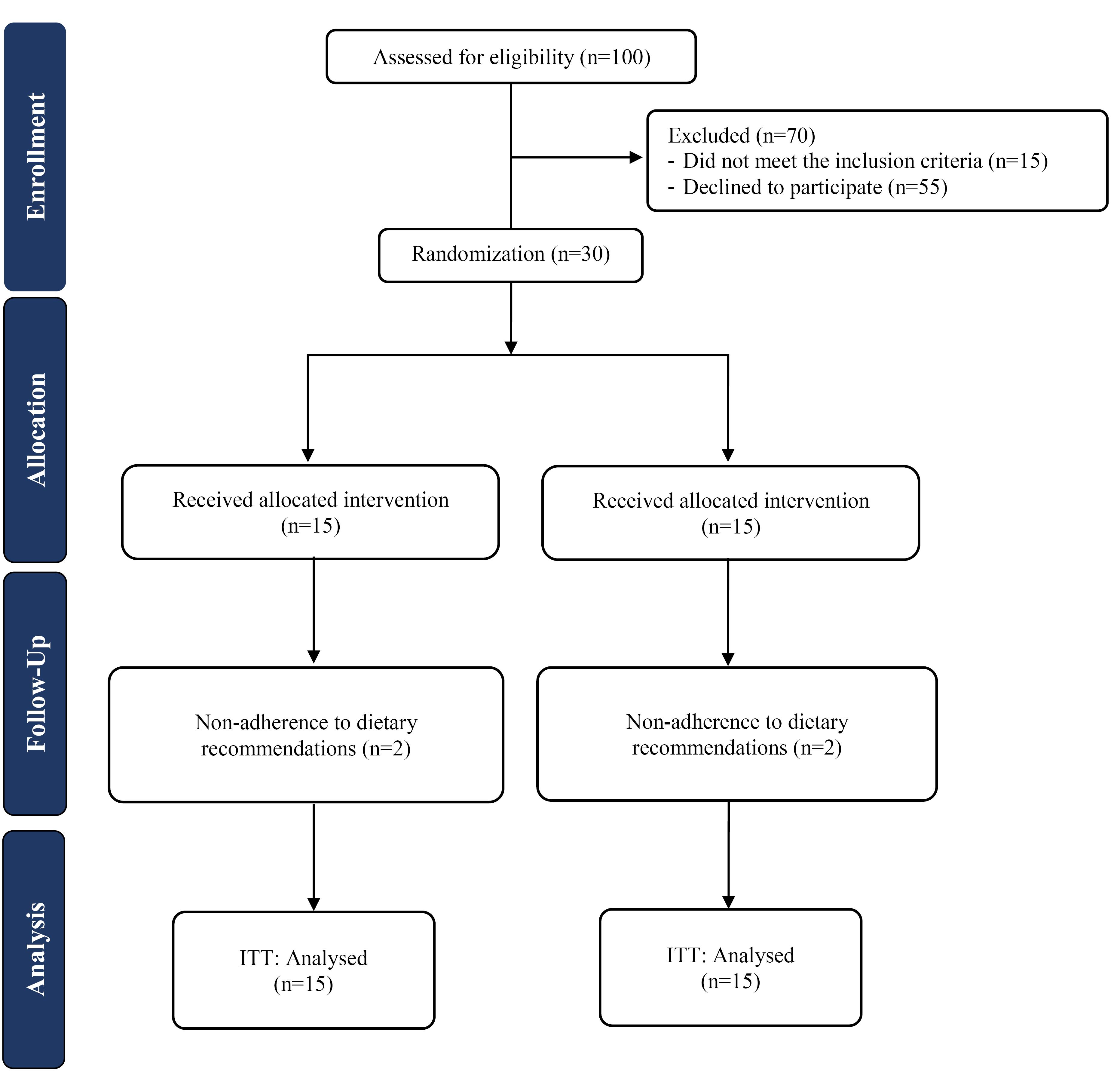
Figure 1.
Study flow diagram. Note. ITT: Intention to treat
.
Study flow diagram. Note. ITT: Intention to treat
Table 1.
Baseline Characteristics of the Study Patients
|
|
Soybean (n=15)
|
Control (n=15)
|
| Age (y) |
34.6 (10.38) |
38.2 (11.04) |
| BMI (kg/m2) |
24.8 (3.89) |
26.7 (6.02) |
| Gender (male), n (%) |
9 (60.0) |
11 (73.3) |
| Education (educated), n (%) |
6 (40) |
9 (60) |
| Marital status (married), n (%) |
10 (66.7) |
12 (80) |
| Physical activity (MET-h/day) |
20.0 (6.22) |
19.6 (9.25) |
| Abdominal pain, n (%) |
5 (33.3) |
2 (13.3) |
| Diarrhea, n (%) |
4 (26.7) |
3 (20) |
| Constipation, n (%) |
0 (0.0) |
1 (6.7) |
Note. BMI: Body mass index; MET: Metabolic equivalent task.
Data are presented as mean (standard deviation) or n (percent).
Obtained fromindependent samples t-test or chi-square test, where appropriate.
Table 2.
Dietary Intake of the Patients Throughout the Study
|
|
Soybean (n=15)
|
Control (n=15)
|
P
valuea
|
| Energy (kcal/d) |
1837 (534) |
1730 (719) |
0.64 |
| Carbohydrate (g/d) |
295 (96.5) |
265 (98.9) |
0.41 |
| Total fat (g/d) |
40.2 (16.3) |
41.6 (30.7) |
0.94 |
| Protein (g/d) |
70.9 (32.6) |
76.4 (37.4) |
0.67 |
| SFA (g/d) |
11.8 (5.3) |
11.6 (7.31) |
0.90 |
| PUFA (g/d) |
11.6 (8.66) |
12.4 (16.5) |
0.87 |
| Dietary fiber (g/d) |
15.9 (5.08) |
12.41 (3.79) |
0.04 |
| Vitamin E (mg/d) |
3.14 (3.30) |
3.71 (2.01) |
0.57 |
| Vitamin C (mg/d) |
66.9 (58.4) |
126 (106) |
0.07 |
| Iron (mg/d) |
15.5 (6.08) |
12.7 (4.71) |
0.18 |
| Calcium (mg/d) |
511 (218) |
600 (374) |
0.43 |
| Magnesium (mg/d) |
217 (46.6) |
207 (91.8) |
0.71 |
| Potassium (mg/d) |
2212 (553) |
2235 (1233) |
0.92 |
Note. PUFA: Polyunsaturated fatty acid; SFA: Saturated fatty acid.
Data are presented as mean (standard deviation).
aObtained fromindependent samples t-test.
Table 3 illustrates no significant differences in study outcomes between the study groups at baseline. Disease severity and quality of life did not differ significantly between the groups at the end of the study, and there were no statistically significant changes in IBD-related disabilities within or between the groups. Additionally, there was no significant effect of soybean consumption on psychological distress and depression. However, soybean consumption did significantly improve anxiety in patients with UC compared to the control group (P = 0.02).
Table 3.
Effect of Soy Intake on Study Outcomes
|
|
Soybean (n=15)
|
Control (n=15)
|
MD
|
95% CI
|
P
-value
|
| Disease severity |
|
|
|
|
|
| Baseline |
3.46 (1.06) |
2.93 (1.03) |
0.53 |
-0.24, 1.31 |
0.17a |
| End |
3.53 (0.91) |
2.86 (1.30) |
0.23 |
-0.46, 0.93 |
0.49c |
| MD |
0.06 |
-0.06 |
|
|
|
| 95% CI |
-0.46, 0.59 |
-0.55, 0.42 |
|
|
|
|
p b
|
0.79 |
0.77 |
|
|
|
| IBD-DI |
|
|
|
|
|
| Baseline |
-6.00 (15.51) |
-3.53 (17.13) |
-2.46 |
-14.69, 9.76 |
0.68a |
| End |
-4.13 (16.92) |
-3.93 (13.36) |
1.34 |
-5.55, 8.24 |
0.69c |
| MD |
1.86 |
-0.40 |
|
|
|
| 95% CI |
-1.95, 5.68 |
-6.87, 6.07 |
|
|
|
|
p b
|
0.31 |
0.89 |
|
|
|
| Quality of life |
|
|
|
|
|
| Baseline |
41.60 (7.70) |
45.06 (8.14) |
-3.46 |
-9.39, 2.46 |
0.24a |
| End |
42.20 (6.61) |
44.26 (6.09) |
-0.27 |
-3.79, 3.24 |
0.87c |
| MD |
0.60 |
-0.80 |
|
|
|
| 95% CI |
-2.37, 3.57 |
-4.00, 2.40 |
|
|
|
|
p b
|
0.67 |
0.60 |
|
|
|
| Psychological distress |
|
|
|
|
|
| Baseline |
48.46 (8.35) |
50.20 (11.97) |
-1.73 |
-9.45, 5.98 |
0.65a |
| End |
49.20 (8.23) |
48.26 (9.70) |
2.25 |
-2.79, 7.24 |
0.37c |
| MD |
0.73 |
-1.93 |
|
|
|
| 95% CI |
-2.74, 4.21 |
-6.66, 2.79 |
|
|
|
|
p b
|
0.65 |
0.39 |
|
|
|
| Depression |
|
|
|
|
|
| Baseline |
9.20 (3.60) |
10.46 (2.58) |
-1.26 |
-3.61, 1.08 |
0.28a |
| End |
8.93 (4.43) |
9.86 (2.99) |
0.23 |
-2.05, 2.52 |
0.83c |
| MD |
-0.26 |
-0.60 |
|
|
|
| 95% CI |
-1.86, 1.33 |
-2.39, 1.19 |
|
|
|
|
p b
|
0.72 |
0.48 |
|
|
|
| Anxiety |
|
|
|
|
|
| Baseline |
9.06 (3.71) |
9.93 (2.37) |
-0.86 |
-3.19, 1.46 |
0.45a |
| End |
8.00 (3.52) |
10.86 (2.53) |
-2.43 |
-4.45, -0.42 |
0.02c |
| MD |
-1.06 |
0.93 |
|
|
|
| 95% CI |
-2.89, 0.76 |
-0.41, 2.28 |
|
|
|
|
p b
|
0.23 |
0.16 |
|
|
|
Note. MD: Mean of difference; CI: Confidence interval; IBD-DI: Inflammatory bowel disease disability index.
Data are presented as mean (standard deviation).
aObtained fromindependent samples t-test.
bObtained from paired sample t-test.
cObtained fromANCOVA adjusted for age and baseline values.
Discussion
In the current study, soybean consumption was found to significantly improve anxiety in patients with UC, while no significant changes were observed in terms of quality of life, disease severity, IBD-related disabilities, depression, and psychological distress.
UC is a prevalent chronic gastrointestinal disease.The clinical symptoms of IBD, including diarrhea, abdominal pain, fatigue, and bleeding, are characterized by periods of relapse and remission. Long-standing inflammation in IBD can lead to the development of colorectal cancer.30 First-line therapies for IBD typically involve medications such as 5-aminosalicylates, corticosteroids, and immunosuppressive drugs. While these medications are often effective, long-term usage can result in adverse side effects.31-33 Maintaining remission in IBD is a clinical challenge that is currently under-researched. Evidence suggests that diet plays a crucial role in managing the severity of IBD and improving quality of life. Soybean, known for its anti-inflammatory and immunomodulatory properties, is considered a good option for inflammatory diseases such as IBD. Previous studies have reported the beneficial effects of consuming soybean components on patients with IBD. However, these effects have not yet been assessed with soybean. This study is the first clinical trial to investigate the effects of soybean consumption on disease severity, IBD-related disabilities, quality of life, and mental health in patients with UC. In the current study, soybean consumption had no significant effect on the severity of UC. Consistent with our finding, results of a randomized clinical trial showed that supplementation with soy and whey protein for 16 weeks does not affect CD disease activity.34 Jalili et al35 also reported that simultaneous use of soy isoflavone and vitamin D supplement does not affect the severity of symptoms of irritable bowel disease.
In contrast, daily consumption of 30 grams of flaxseed or 20 grams of flaxseed oil for 12 weeks significantly reduced the severity of UC.36 The conflicting results in these studies may be due to differences in the content of bioactive compounds in soybean and flaxseed products. For example, the predominant polyunsaturated fatty acid (PUFA) in flaxseed is alpha-linolenic acid, while the predominant PUFA in soybeans is linoleic acid. In addition, difference in the duration of intervention could be another reason for the conflicting results of the studies.
In the present study, an increase in the incidence of diarrhea was observed in the soybean group compared to the control group. This finding is consistent with results of a study in which consumption of soy milk, compared to yogurt or hydrolyzed formula, in infants with chronic diarrhea non-significantly increased the severity of diarrhea.37 In another study, consuming 200 mg of soy isoflavones for two years had no significant effect on reducing constipation in postmenopausal women. Additionally, consumption of soy isoflavones did not reduce headaches, bloating, and dry mouth in postmenopausal women.38 Unlike our finding, administration of soy-based formulas in healthy infants who were intolerant to milk-based formulas significantly reduced gastrointestinal symptoms.39 The increased diarrhea associated with soy consumption is not well understood. One hypothesis is that the presence of short-chain carbohydrates in the carbohydrate composition of soybean can cause this effect. Furthermore, short-chain carbohydrates play an important role in creating osmotic pressure in the intestine, leading to diarrhea.
The finding of the present study showed that soybean consumption, compared to the control group, does not improve the quality of life in patients with UC. Consistent with our finding, Nourozi et al reported that daily consumption of 500 mL of soy milk for 8 weeks does not affect the quality of life of postmenopausal women.40 Our finding was also consistent with another clinical trial, which indicated that supplementation with soy isoflavones and vitamin D for 6 weeks does not improve the quality of life in patients with irritable bowel syndrome.35 In another study, supplementation with soy isoflavones had no significant effect on the quality of life of postmenopausal women.41 Nevertheless, our finding contrast with a randomized clinical trial that reported supplementation with fermented soybeans for three weeks significantly improves the quality of life in adults experiencing heartburn.42 These conflicting results might be explained by differences in the duration of studies and the evaluation of individuals with different clinical conditions across studies.
Our finding displayed that the consumption of soybean does not significantly affect the disability caused by UC. Results of a case-series study showed that adherence to an anti-inflammatory diet, which also contains soy products, can reduce fatigue, bloating, diarrhea, and pain in patients with IBD, all of which contribute to the disabilities caused by the disease.43 An underlying cause for the difference in the findings of this study and our results could be related to the different designs of the interventions. For example, in the case-series study, soy products were given to participants as part of an anti-inflammatory diet, and the presence of other anti-inflammatory compounds could have also reduced the mentioned symptoms.
Our findings demonstrated that soybean consumption significantly impacts anxiety in patients with UC. However, it had no significant effect on the levels of depression and psychological distress of the patients. A cross-sectional study showed that legume consumption has an inverse and significant relationship with anxiety in adults, while legume consumption was not associated with psychological stress in this study.44 Another study reported a non-significant association between adherence to a diet rich in soy or its products and psychological stress.45 In the study by Balk et al, daily consumption of 100 mg of soy isoflavone for 6 months did not have a significant effect on depressive symptoms in postmenopausal women.46 In contrast, consumption of soybeans for 3 months in 40 depressed postmenopausal women improved depression and increased response to antidepressants.47 These conflicting results of studies may be due to differences in mental health assessment tools, participants with different clinical conditions, and different durations of interventions across studies. In addition, the lack of effect of soybean on depression and psychological distress could be due to the short duration of the intervention, as available evidence suggests that depression is affected later than anxiety in interventions.48 Overall, clinical trials with longer intervention durations are needed to draw definitive results.
In the literature, several mechanisms have been proposed for the anticolitic effects of soy. Soy is rich in phytoestrogens, phospholipids, phytosterols, saponins, and bioactive peptides. Isoflavones, for instance, are believed to function as antioxidants in patients with colitis by directly scavenging free radicals and increasing antioxidant enzyme systems.49,50 Phytosterols, despite being well-known for their cholesterol-lowering effects, also exhibit anti-inflammatory properties.51 β-Sitosterol, the most abundant phytosterol in soybeans, has immunomodulatory properties,52 and soyasaponins, though not highly bioavailable orally, were found to be beneficial to the colon.53,54 In a mouse model of colon tumorigenesis, crude soyasaponin extracts exhibited anticancer potential and antioxidant properties. Soyasaponins also demonstrated anti-inflammatory effects in vitro and in mouse models of intestinal inflammation.55,56 Additionally, an early study in rats revealed that rectal administration of glycerophospholipids improves histological signs of colitis and reduces the permeability of the colonic mucosa.57 The therapeutic potential of phosphatidylcholine in IBD was supported by studies showing that patients with UC in remission had lower levels of phosphatidylcholine and lysophosphatidylcholine (an intermediate of phospholipase A2) in rectal mucus samples.58,59
Strengths
To the best of our knowledge, it seems that the present study is the first to investigate the effect of soybean consumption on individuals with UC.Several outcomes were examined in patients at the study baseline and at the end of the trial. Using the ITT approach and including all randomized subjects allowed us to obtain an unbiased estimate of the intervention’s effect. Moreover, adherence to the intervention and information about participants’ food intake and physical activity were assessed throughout the study.
Limitations
The duration of the study might not have been long enough to affect some of the study outcomes. Although the study groups were well-balanced for baseline characteristics, potential differences in unknown confounders between the groups cannot be ruled out. In this study, a single dose of soy was investigated, preventing the establishment of a dose-response relationship. Funding restrictions prevented us from measuring urine levels of isoflavonoids to assess patient compliance. Due to the relatively small sample size, we were unable to examine the effect of soy consumption on study outcomes stratified by gender or other demographic variables. Furthermore, the results may not be generalizable to patients with CD. Financial constraints also prevented us from investigating the effects of soy intake on inflammatory biomarkers. Finally, due to the nature of the food intervention in the present study, it was not possible to blind study participants, which could affect the findings. Larger trials are needed to address these limitations and further explore the effects of soy intake on study outcomes, stratified by gender or other demographic variables.
Conclusion
In summary, soybean consumption significantly improved anxiety of patients with UC, while no significant changes were observed in terms of quality of life, disease severity, IBD-related disabilities, depression, and psychological distress. To confirm our findings, further studies are required.
Ethics statement
The study design was approved by the Bioethics Committee of Tehran University of Medical Sciences, Tehran, Iran (identifier: IR.TUMS.VCR.REC.1398.973).
Conflict of interests declaration
The authors declare no conflict of interests.
Author contributions
Conceptualization: Sina Naghshi, Ahmad Esmaillzadeh, Leila Azadbakht, Seyed Ali Keshavarz, Amir Ali Sohrabpour.
Data curation: Sina Naghshi.
Formal analysis: Sina Naghshi, Helda Tutunchi, Omid Sadeghi, Amir Ali Sohrabpour.
Funding acquisition: Ahmad Esmaillzadeh.
Investigation: Sina Naghshi, Manouchehr Khoshbaten, Omid Sadeghi.
Methodology: Sina Naghshi, Ahmad Esmaillzadeh, Leila Azadbakht, Seyed Ali Keshavarz.
Project administration: Sina Naghshi, Ahmad Esmaillzadeh, Leila Azadbakht, Seyed Ali Keshavarz.
Resources: Sina Naghshi, Helda Tutunchi, Amir Ali Sohrabpour, Manouchehr Khoshbaten.
Software: Sina Naghshi.
Supervision: Ahmad Esmaillzadeh.
Validation: Sina Naghshi, Ahmad Esmaillzadeh.
Visualization: Sina Naghshi, Seyed Ali Keshavarz.
Writing–original draft: Sina Naghshi, Helda Tutunchi, Sevil Kiani, Omid Sadeghi.
Writing–review & editing: Ahmad Esmaillzadeh, Leila Azadbakht, Amir Ali Sohrabpour.
References
- Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet 2007; 369(9573):1627-40. doi: 10.1016/s0140-6736(07)60750-8 [Crossref] [ Google Scholar]
- Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis 2006; 12 Suppl 1:S3-9. doi: 10.1097/01.mib.0000195385.19268.68 [Crossref] [ Google Scholar]
- Malekzadeh MM, Vahedi H, Gohari K, Mehdipour P, Ghajarieh Sepanlou S, Ebrahimi Daryani N. Emerging epidemic of inflammatory bowel disease in a middle-income country: a nation-wide study from Iran. Arch Iran Med 2016; 19(1):2-15. [ Google Scholar]
- Dolan KT, Chang EB. Diet, gut microbes, and the pathogenesis of inflammatory bowel diseases. Mol Nutr Food Res 2017;61(1):10.1002/mnfr.201600129. doi: 10.1002/mnfr.201600129.
- Shivashankar R, Lewis JD. The role of diet in inflammatory bowel disease. Curr Gastroenterol Rep 2017; 19(5):22. doi: 10.1007/s11894-017-0563-z [Crossref] [ Google Scholar]
- Jonkers D, Penders J, Masclee A, Pierik M. Probiotics in the management of inflammatory bowel disease: a systematic review of intervention studies in adult patients. Drugs 2012; 72(6):803-23. doi: 10.2165/11632710-000000000-00000 [Crossref] [ Google Scholar]
- Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol 2011; 106(4):563-73. doi: 10.1038/ajg.2011.44 [Crossref] [ Google Scholar]
- Racine A, Carbonnel F, Chan SS, Hart AR, Bueno-de-Mesquita HB, Oldenburg B. Dietary patterns and risk of inflammatory bowel disease in Europe: results from the EPIC study. Inflamm Bowel Dis 2016; 22(2):345-54. doi: 10.1097/mib.0000000000000638 [Crossref] [ Google Scholar]
- Swanson GR, Sedghi S, Farhadi A, Keshavarzian A. Pattern of alcohol consumption and its effect on gastrointestinal symptoms in inflammatory bowel disease. Alcohol 2010; 44(3):223-8. doi: 10.1016/j.alcohol.2009.10.019 [Crossref] [ Google Scholar]
- Barthel C, Wiegand S, Scharl S, Scharl M, Frei P, Vavricka SR. Patients’ perceptions on the impact of coffee consumption in inflammatory bowel disease: friend or foe?--A patient survey. Nutr J 2015; 14:78. doi: 10.1186/s12937-015-0070-8 [Crossref] [ Google Scholar]
- MacDermott RP. Treatment of irritable bowel syndrome in outpatients with inflammatory bowel disease using a food and beverage intolerance, food and beverage avoidance diet. Inflamm Bowel Dis 2007; 13(1):91-6. doi: 10.1002/ibd.20048 [Crossref] [ Google Scholar]
- Del Pinto R, Pietropaoli D, Chandar AK, Ferri C, Cominelli F. Association between inflammatory bowel disease and vitamin D deficiency: a systematic review and meta-analysis. Inflamm Bowel Dis 2015; 21(11):2708-17. doi: 10.1097/mib.0000000000000546 [Crossref] [ Google Scholar]
- Oldenburg B, Koningsberger JC, Van Berge Henegouwen GP, Van Asbeck BS, Marx JJ. Iron and inflammatory bowel disease. Aliment Pharmacol Ther 2001; 15(4):429-38. doi: 10.1046/j.1365-2036.2001.00930.x [Crossref] [ Google Scholar]
- Juritsch AF, Moreau R. Role of soybean-derived bioactive compounds in inflammatory bowel disease. Nutr Rev 2018; 76(8):618-38. doi: 10.1093/nutrit/nuy021 [Crossref] [ Google Scholar]
- Bitzer ZT, Wopperer AL, Chrisfield BJ, Tao L, Cooper TK, Vanamala J. Soy protein concentrate mitigates markers of colonic inflammation and loss of gut barrier function in vitro and in vivo. J Nutr Biochem 2017; 40:201-8. doi: 10.1016/j.jnutbio.2016.11.012 [Crossref] [ Google Scholar]
- Lichtenstein GR, Deren JJ, Katz S, Lewis JD, Kennedy AR, Ware JH. Bowman-Birk inhibitor concentrate: a novel therapeutic agent for patients with active ulcerative colitis. Dig Dis Sci 2008; 53(1):175-80. doi: 10.1007/s10620-007-9840-2 [Crossref] [ Google Scholar]
- Masnadi Shirazi K, Nikniaz Z, Masnadi Shirazi A, Rohani M. Vitamin A supplementation decreases disease activity index in patients with ulcerative colitis: a randomized controlled clinical trial. Complement Ther Med 2018; 41:215-9. doi: 10.1016/j.ctim.2018.09.026 [Crossref] [ Google Scholar]
- Forbes A, Escher J, Hébuterne X, Kłęk S, Krznaric Z, Schneider S. ESPEN guideline: clinical nutrition in inflammatory bowel disease. Clin Nutr 2017; 36(2):321-47. doi: 10.1016/j.clnu.2016.12.027 [Crossref] [ Google Scholar]
- Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis 2008; 14(12):1660-6. doi: 10.1002/ibd.20520 [Crossref] [ Google Scholar]
- Su C, Lewis JD, Goldberg B, Brensinger C, Lichtenstein GR. A meta-analysis of the placebo rates of remission and response in clinical trials of active ulcerative colitis. Gastroenterology 2007; 132(2):516-26. doi: 10.1053/j.gastro.2006.12.037 [Crossref] [ Google Scholar]
- Peyrin-Biroulet L, Cieza A, Sandborn WJ, Coenen M, Chowers Y, Hibi T. Development of the first disability index for inflammatory bowel disease based on the international classification of functioning, disability and health. Gut 2012; 61(2):241-7. doi: 10.1136/gutjnl-2011-300049 [Crossref] [ Google Scholar]
- Leong RW, Huang T, Ko Y, Jeon A, Chang J, Kohler F. Prospective validation study of the International Classification of Functioning, Disability and Health score in Crohn’s disease and ulcerative colitis. J Crohns Colitis 2014; 8(10):1237-45. doi: 10.1016/j.crohns.2014.02.028 [Crossref] [ Google Scholar]
- Verissimo R. Quality of life in inflammatory bowel disease: psychometric evaluation of an IBDQ cross-culturally adapted version. J Gastrointestin Liver Dis 2008; 17(4):439-44. [ Google Scholar]
- Neuendorf R, Harding A, Stello N, Hanes D, Wahbeh H. Depression and anxiety in patients with inflammatory bowel disease: a systematic review. J Psychosom Res 2016; 87:70-80. doi: 10.1016/j.jpsychores.2016.06.001 [Crossref] [ Google Scholar]
- Byrne G, Rosenfeld G, Leung Y, Qian H, Raudzus J, Nunez C. Prevalence of anxiety and depression in patients with inflammatory bowel disease. Can J Gastroenterol Hepatol 2017; 2017:6496727. doi: 10.1155/2017/6496727 [Crossref] [ Google Scholar]
- Montazeri A, Vahdaninia M, Ebrahimi M, Jarvandi S. The Hospital Anxiety and Depression Scale (HADS): translation and validation study of the Iranian version. Health Qual Life Outcomes 2003; 1:14. doi: 10.1186/1477-7525-1-14 [Crossref] [ Google Scholar]
- Schmitz N, Kruse J, Heckrath C, Alberti L, Tress W. Diagnosing mental disorders in primary care: the General Health Questionnaire (GHQ) and the Symptom Check List (SCL-90-R) as screening instruments. Soc Psychiatry Psychiatr Epidemiol 1999; 34(7):360-6. doi: 10.1007/s001270050156 [Crossref] [ Google Scholar]
- Taghavi SM. Validity and reliability of the General Health Questionnaire (GHQ-28) in college students of Shiraz university. J Psychol 2002;5(4):381-98. [Persian].
- Noorbala AA, Bagheri Yazdi SH, Mohammad K. The validation of General Health Questionnaire-28 as a psychiatric screening tool. Hakim Res J 2009;11(4):47-53. [Persian].
- Dulai PS, Sandborn WJ, Gupta S. Colorectal cancer and dysplasia in inflammatory bowel disease: a review of disease epidemiology, pathophysiology, and management. Cancer Prev Res (Phila) 2016; 9(12):887-94. doi: 10.1158/1940-6207.Capr-16-0124 [Crossref] [ Google Scholar]
- Scheman A, Te R. Contact allergy to salicylates and cross-reactions. Dermatitis 2017; 28(4):291. doi: 10.1097/der.0000000000000300 [Crossref] [ Google Scholar]
- Zmudzinska M, Czarnecka-Operacz M, Silny W. Contact allergy to glucocorticosteroids in patients with chronic venous leg ulcers, atopic dermatitis and contact allergy. Acta Dermatovenerol Croat 2008; 16(2):72-8. [ Google Scholar]
- Wypych TP, Marsland BJ. Antibiotics as instigators of microbial dysbiosis: implications for asthma and allergy. Trends Immunol 2018; 39(9):697-711. doi: 10.1016/j.it.2018.02.008 [Crossref] [ Google Scholar]
- Machado JF, Oya V, Coy CS, Morcillo AM, Severino SD, Wu C. Whey and soy protein supplements changes body composition in patients with Crohn’s disease undergoing azathioprine and anti-TNF-alpha therapy. Nutr Hosp 2015; 31(4):1603-10. doi: 10.3305/nh.2015.31.4.8362 [Crossref] [ Google Scholar]
- Jalili M, Hekmatdoost A, Vahedi H, Poustchi H, Khademi B, Saadi M. Co-administration of soy isoflavones and vitamin D in management of irritable bowel disease. PLoS One 2016; 11(8):e0158545. doi: 10.1371/journal.pone.0158545 [Crossref] [ Google Scholar]
- Morshedzadeh N, Shahrokh S, Asadzadeh Aghdaei H, Pourhoseingholi MA, Chaleshi V, Hekmatdoost A. Effects of flaxseed and flaxseed oil supplement on serum levels of inflammatory markers, metabolic parameters and severity of disease in patients with ulcerative colitis. Complement Ther Med 2019; 46:36-43. doi: 10.1016/j.ctim.2019.07.012 [Crossref] [ Google Scholar]
- de Mattos AP, Ribeiro TC, Mendes PS, Valois SS, Mendes CM, Ribeiro HC Jr. Comparison of yogurt, soybean, casein, and amino acid-based diets in children with persistent diarrhea. Nutr Res 2009; 29(7):462-9. doi: 10.1016/j.nutres.2009.06.005 [Crossref] [ Google Scholar]
- Levis S, Strickman-Stein N, Ganjei-Azar P, Xu P, Doerge DR, Krischer J. Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms: a randomized, double-blind trial. Arch Intern Med 2011; 171(15):1363-9. doi: 10.1001/archinternmed.2011.330 [Crossref] [ Google Scholar]
- Lasekan JB, Baggs GE. Efficacy of soy-based formulas in alleviating gastrointestinal symptoms in infants with milk-based formula intolerance: a randomized clinical trial. Clin Pediatr (Phila) 2021; 60(3):184-92. doi: 10.1177/0009922820973017 [Crossref] [ Google Scholar]
- Nourozi M, Haghollahi F, Ramezanzadeh F, Hanachi P. Effect of soy milk consumption on quality of life in Iranian postmenopausal women. J Family Reprod Health 2015; 9(2):93-100. [ Google Scholar]
- Amato P, Young RL, Steinberg FM, Murray MJ, Lewis RD, Cramer MA. Effect of soy isoflavone supplementation on menopausal quality of life. Menopause 2013; 20(4):443-7. doi: 10.1097/gme.0b013e318275025e [Crossref] [ Google Scholar]
- Fatani A, Vaher K, Rivero-Mendoza D, Alabasi K, Dahl WJ. Fermented soy supplementation improves indicators of quality of life: a randomized, placebo-controlled, double-blind trial in adults experiencing heartburn. BMC Res Notes 2020; 13(1):364. doi: 10.1186/s13104-020-05205-z [Crossref] [ Google Scholar]
- Olendzki BC, Silverstein TD, Persuitte GM, Ma Y, Baldwin KR, Cave D. An anti-inflammatory diet as treatment for inflammatory bowel disease: a case series report. Nutr J 2014; 13:5. doi: 10.1186/1475-2891-13-5 [Crossref] [ Google Scholar]
- Anjom-Shoae J, Sadeghi O, Hassanzadeh Keshteli A, Afshar H, Esmaillzadeh A, Adibi P. Legume and nut consumption in relation to depression, anxiety and psychological distress in Iranian adults. Eur J Nutr 2020; 59(8):3635-45. doi: 10.1007/s00394-020-02197-1 [Crossref] [ Google Scholar]
- Ebrahimpour-Koujan S, Hassanzadeh Keshteli A, Afshar H, Esmaillzadeh A, Adibi P. Adherence to low carbohydrate diet and prevalence of psychological disorders in adults. Nutr J 2019; 18(1):87. doi: 10.1186/s12937-019-0513-8 [Crossref] [ Google Scholar]
- Balk JL, Whiteside DA, Naus G, DeFerrari E, Roberts JM. A pilot study of the effects of phytoestrogen supplementation on postmenopausal endometrium. J Soc Gynecol Investig 2002; 9(4):238-42. [ Google Scholar]
- Estrella RE, Landa AI, Lafuente JV, Gargiulo PA. Effects of antidepressants and soybean association in depressive menopausal women. Acta Pol Pharm 2014; 71(2):323-7. [ Google Scholar]
- Schueller SM, Aguilera A, Mohr DC. Ecological momentary interventions for depression and anxiety. Depress Anxiety 2017; 34(6):540-5. doi: 10.1002/da.22649 [Crossref] [ Google Scholar]
- Rüfer CE, Kulling SE. Antioxidant activity of isoflavones and their major metabolites using different in vitro assays. J Agric Food Chem 2006; 54(8):2926-31. doi: 10.1021/jf053112o [Crossref] [ Google Scholar]
- Ruiz-Larrea MB, Mohan AR, Paganga G, Miller NJ, Bolwell GP, Rice-Evans CA. Antioxidant activity of phytoestrogenic isoflavones. Free Radic Res 1997; 26(1):63-70. doi: 10.3109/10715769709097785 [Crossref] [ Google Scholar]
- Othman RA, Moghadasian MH. Beyond cholesterol-lowering effects of plant sterols: clinical and experimental evidence of anti-inflammatory properties. Nutr Rev 2011; 69(7):371-82. doi: 10.1111/j.1753-4887.2011.00399.x [Crossref] [ Google Scholar]
- Kim KA, Lee IA, Gu W, Hyam SR, Kim DH. β-Sitosterol attenuates high-fat diet-induced intestinal inflammation in mice by inhibiting the binding of lipopolysaccharide to toll-like receptor 4 in the NF-κB pathway. Mol Nutr Food Res 2014; 58(5):963-72. doi: 10.1002/mnfr.201300433 [Crossref] [ Google Scholar]
- Kawamori T, Tanaka T, Hara A, Yamahara J, Mori H. Modifying effects of naturally occurring products on the development of colonic aberrant crypt foci induced by azoxymethane in F344 rats. Cancer Res 1995; 55(6):1277-82. [ Google Scholar]
- Koratkar R, Rao AV. Effect of soya bean saponins on azoxymethane-induced preneoplastic lesions in the colon of mice. Nutr Cancer 1997; 27(2):206-9. doi: 10.1080/01635589709514526 [Crossref] [ Google Scholar]
- Kang JH, Sung MK, Kawada T, Yoo H, Kim YK, Kim JS. Soybean saponins suppress the release of proinflammatory mediators by LPS-stimulated peritoneal macrophages. Cancer Lett 2005; 230(2):219-27. doi: 10.1016/j.canlet.2004.12.041 [Crossref] [ Google Scholar]
- Lee IA, Park YJ, Yeo HK, Han MJ, Kim DH. Soyasaponin I attenuates TNBS-induced colitis in mice by inhibiting NF-κB pathway. J Agric Food Chem 2010; 58(20):10929-34. doi: 10.1021/jf102296y [Crossref] [ Google Scholar]
- Fabia R, Ar’Rajab A, Willén R, Andersson R, Ahrén B, Larsson K. Effects of phosphatidylcholine and phosphatidylinositol on acetic-acid-induced colitis in the rat. Digestion 1992; 53(1-2):35-44. doi: 10.1159/000200969 [Crossref] [ Google Scholar]
- Ehehalt R, Wagenblast J, Erben G, Lehmann WD, Hinz U, Merle U. Phosphatidylcholine and lysophosphatidylcholine in intestinal mucus of ulcerative colitis patients a quantitative approach by nanoelectrospray-tandem mass spectrometry. Scand J Gastroenterol 2004; 39(8):737-42. doi: 10.1080/00365520410006233 [Crossref] [ Google Scholar]
- Stremmel W, Ehehalt R, Autschbach F, Karner M. Phosphatidylcholine for steroid-refractory chronic ulcerative colitis: a randomized trial. Ann Intern Med 2007; 147(9):603-10. doi: 10.7326/0003-4819-147-9-200711060-00004 [Crossref] [ Google Scholar]