Int J Drug Res Clin. 2:e1.
doi: 10.34172/ijdrc.2024.e1
Review Article
The Nephrotoxicity of Checkpoint Inhibitors: A Mini Review
Fariba Mahmoodpoor 1  , Mohammadreza Ardalan 2, Aygun Nasibova 3, Mahbuba Valiyeva 4, Elham Ahmadian 1, *
, Mohammadreza Ardalan 2, Aygun Nasibova 3, Mahbuba Valiyeva 4, Elham Ahmadian 1, * 
Author information:
1Research Center for Integrative Medicine in Aging, Aging Research Institute,Tabriz University of Medical Sciences, Tabriz, Iran
2Kidney Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
3Department of Biophysics and Biochemistry, Baku State University, Baku, Azerbaijan
4Department of Pharmaceutical Technology and Management, Azerbaijan Medical University, Baku 1022, Azerbaijan
Abstract
Immune checkpoint inhibitors (ICIs) have established promising therapeutic outcomes in the treatment of more than 17 tumors and are being used more frequently. Although these drugs have made progress in cancer treatment over the past ten years, severe immune-related adverse events (irAEs) can be fatal. Nephrotoxicity is less frequent than toxicities that affect the gastrointestinal tract, the skin, and the endocrine system, yet it is frequently underdiagnosed because of its difficulty. The most frequent nephrotoxicity is acute kidney injury (AKI), which is typically linked to acute interstitial nephritis. It is possible for kidney impairment caused by ICIs to manifest in much more varied ways, which may have an impact on the management strategies for therapeutic as well as diagnostic purposes. The current study discussed the most recent ICIs for cancer that have been approved, the risk factors and prevalence of nephrotoxicity, the present knowledge of the pathological mechanisms of AKI, and the management protocols.
Keywords: Nephrotoxicity, Acute kidney injury, Cancer, Checkpoint inhibitor
Copyright and License Information
© 2024 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Mahmoodpoor F, Ardalan M, Nasibova A, Mahbuba Valiyeva, Ahmadian E. The nephrotoxicity of checkpoint inhibitors: a mini review. Int J Drug Res Clin. 2024;2: e1. doi: 10.34172/ijdrc.2024.e1
Introduction
Immune checkpoint inhibitors (ICIs) are monoclonal antibodies developed to control the negative feedback loop of the immune system by blocking the surface receptors that are primarily found on immune cells such as B and T cells and some tumor cells.1,2 ICIs can prohibit or/and take away the brakes from the immune system by attaching to these receptors. As a result, ICIs increase T cell activation and have antitumor effects.3 The CD28 homolog CTLA-4 or CD152 is a protein mostly expressed in T cells and is allied with cytotoxic T cells4 (Figure 1).
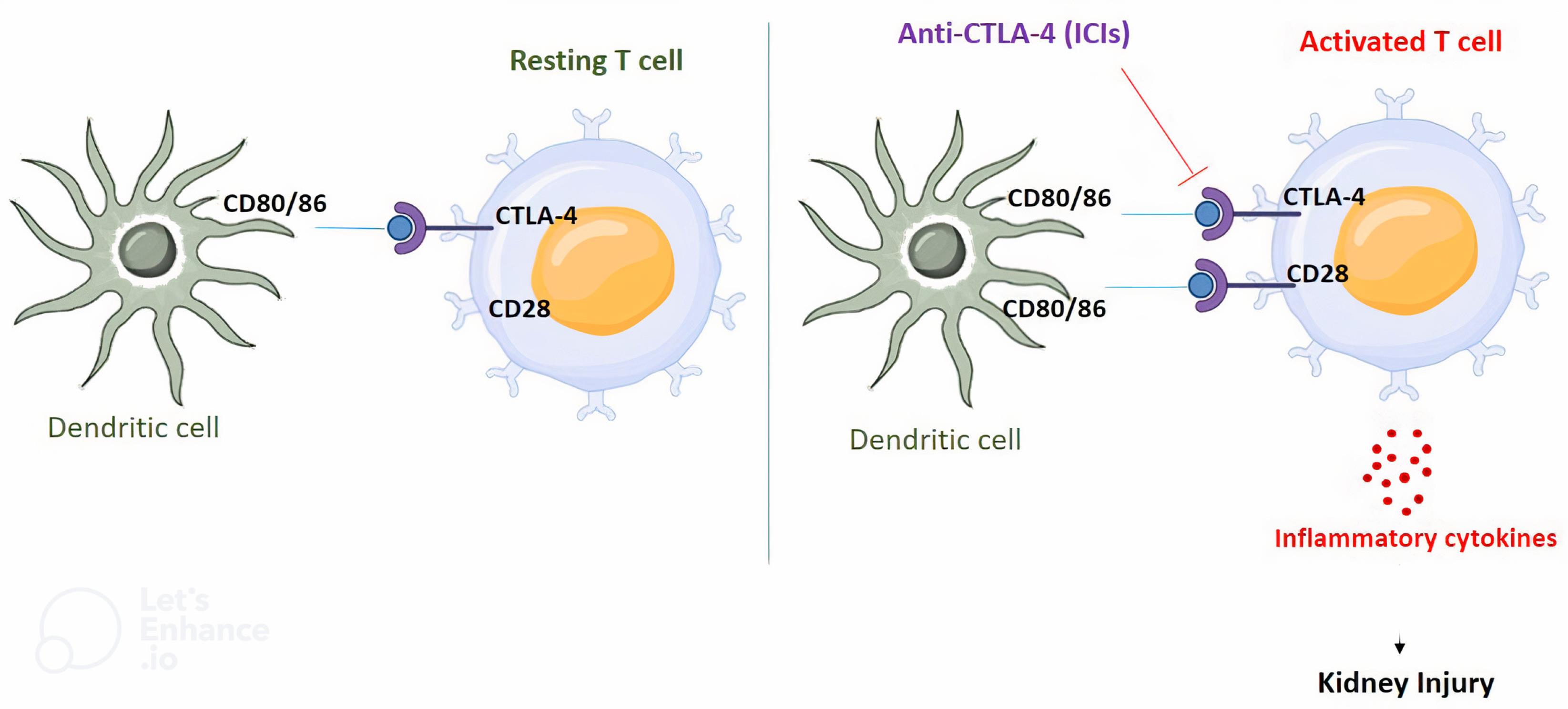
Figure 1.
The Possible Mechanism of Nephrotoxicity Induced by ICIs. Note. ICI: Immune checkpoint inhibitors; In not activated T cells, CTLA-4 binds to CD80/026 on dendritic cell competing with CD28. The use of ICIs blocks this interaction and stimulates the binding of CD28 to CD80/86 which in turn activates T cells, resulting in the release of inflammatory cytokines that are plausible causes of nephrotoxicity
.
The Possible Mechanism of Nephrotoxicity Induced by ICIs. Note. ICI: Immune checkpoint inhibitors; In not activated T cells, CTLA-4 binds to CD80/026 on dendritic cell competing with CD28. The use of ICIs blocks this interaction and stimulates the binding of CD28 to CD80/86 which in turn activates T cells, resulting in the release of inflammatory cytokines that are plausible causes of nephrotoxicity
B7 (CD80/86) is mostly found on antigen-presenting cells, and CTLA-4 has a high affinity for it. This affinity can reduce and/or impede the activation of T cells.5 The U.S. Food and Drug Administration (FDA) approved the initial ICI medication, ipilimumab, a CTLA-4 inhibitor, for the treatment of melanomas in 2011.6 Six ICIs, including protein 1 (PD-1) (cemiplimab, pembrolizumab, nivolumab), or PD-ligand 1 (PD-L1) (atezolizumab, durvalumab, and avelumab), that target programmed cell death have been approved by the FDA after ipilimumab was approved.7 The incidence of anti-PD-1-mediated nephrotoxicity is significantly higher in all grades compared to control groups.8
Only one-third of patients receive benefits from immunotherapy, which has replaced chemotherapy as the treatment of choice for some cancers, while the other two-thirds endure unfavorable, and occasionally fatal, side effects.9 These negative consequences, also known as immune-related adverse events (irAEs), are brought on by autoimmune conditions that can possibly affect all tissues and range in severity. The most frequent and manageable irAEs are cutaneous, gastrointestinal, and endocrine dysfunctions. Other organs such as kidneys are generally less affected but are more challenging to diagnose and treat.10 The adverse effects are related to the type of ICIs, and the severity and frequency with anti-CTLA4 antibodies (particularly ipilimumab) are higher.11 A small percentage of patients have acute kidney injury (AKI), which typically results from acute interstitial nephritis related to ICIs (ICI-AKI).12
AKI concomitant with proteinuria may be observed. The most common lesion observed on kidney biopsy is tubulointerstitial nephritis and glomerular lesions, while acute tubular injury occurrence is less common. Kidney biopsy is necessary in the majority of patients for the definitive diagnosis and therapeutic guide of the renal lesion.13,14
Electrolytic disorders and imbalances such as hypokalemia, hypocalcemia, hyponatremia, and Fanconi syndrome necessitate close observation to prevent potentially fatal side effects. Steroid administration and/or ICI interruption are the pipelines of renal irAEs treatment since they help avoid irrecoverable organ damage.13 The management of ICI nephrotoxicity is important for the quality of life in cancer cases and their survival.15 The present review represented the most recent data on ICI-AKI incidence, risk factors, and treatment options while assessing potential management scenarios.
Nephrotoxicity Related to Immune Checkpoint Inhibitors
The frequency of nephrotoxicity with ICI treatment has been studied in a few systematic reviews and meta-analyses.16 In cancer therapy, the severity rating system for adverse events identified as the Common Terminology Criteria for Adverse Events (CTCAE) is widely acknowledged. Depending on the rise of serum creatinine (sCr) above the reference range, this system determines five classes of AKI.17 However, frequently decreased muscle mass is observed in cancer cases, which can affect how any surge in creatinine is perceived. The work group of the Kidney Disease Improving Global Outcomes (KDIGO) consensus, on the other hand, categorizes AKI into three stages based on sCr changes.17
The incidence of AKI was 2.2% overall and 0.6% for grade 3 AKI in a pooled study of 3695 patients who received ICIs in phase II and III clinical trials.18 According to a meta-analysis of 4070 patients, those using ICIs had a higher likelihood of developing all-grade nephrotoxicity than patients receiving chemotherapy, but in high-grade AKI, there was no difference within the groups.16 In addition, it has been shown that the combined risk of ipilimumab and nivolumab is greater than the risk of ipilimumab or nivolumab alone. In another meta-analysis of 5722 patients, the data were obtained from ten clinical trials in which 9 studies used ICIs as monotherapy, and in one study patients received both ipilimumab and nivolumab. While the prevalence of high-grade renal toxicity was comparable, the incidence of anti-PD-1-mediated kidney adverse effects of all grades was considerably higher compared to controls.19 Furthermore, previous chemotherapy regimens had no impact on the incidence of ICI-AKI, while pembrolizumab was associated with a significantly higher risk of any grade renal events.19 This can be described by the increased risk of renal injury in individuals with urothelial carcinoma who are also on pembrolizumab. Additionally, the post-marketing monitoring of Chen et al revealed an increasing incidence of immune-associated kidney side events between 2011 and 2019.20 Nivolumab monotherapy was connected with a higher percentage of kidney adverse events (33.24%) compared to nivolumab plus ipilimumab combination therapy (23.55%).20 The lag phase between the commencement of ICIs and AKI is longer than that in other more frequently introduced irAEs. In multicenter research, the median time from the initiation of ICIs to the onset of AKI was reported to be 14 weeks.21
A meta-analysis and systematic review, which included 95 studies on about 64 thousand patients, indicated that the simultaneous use of PD-1 and chemotherapy as well as PD-L1 and chemotherapy was related to more severe AKI and more common severe renal complications.22
In a meta-analysis on 5239 patients with advanced renal cell carcinoma, it was determined that combination therapy with ICIs leads to more proteinuria and renal toxicity than standard treatment with sunitinib.23 Moreover, recent results of the outcome of cancer patients who received ICIs and developed AKI were associated with a greater risk of mortality.24 In a cohort study regarding kidney damage caused by ICIs, almost no risk factor and predictor factor for kidney damage was found except for e GFR.25 Table 1 represents the types of nephrotoxicity and clinical manifestations of ICIs in different types of studies.
Table 1.
The Type of Nephrotoxicity and Clinical Outcome after ICI Administration
|
Type of Study
|
Nephrotoxicity Manifestation
|
Outcome
|
Reference
|
| Network meta-analysis |
Immune-related renal toxicity |
ICI administration is associated with an elevated risk of all-grade immune-related renal toxicity |
16
|
| Clinical investigation |
AKI |
Acute tubulointerstitial nephritis, extrarenal irAE |
18
|
| Systematic review |
Renal toxicity |
Immune-related renal toxicity |
19
|
| International multicenter cohort study |
AKI |
Lower baseline valued glomerular filtration rate, the use of proton pump inhibitor, and extrarenal immune-related renal toxicity were each related to a higher risk of AKI |
20
|
| Network meta-analysis |
AKI, hypertension, chronic kidney injury |
The combination of programmed cell death ligand 1 + ICI was concomitant with an increased rate of kidney events |
22
|
| Meta-analysis |
Nephrotoxicity |
Increased creatinine and proteinuria compared with sunitinib |
23
|
Note. ICI: Immune checkpoint inhibitor; AKI: Acute kidney injury; irAE: Immune-related adverse event.
Pathophysiology of Immune Checkpoint Inhibitor-related Nephrotoxicity
The exact mechanisms underlying ICI-associated AKI are still not fully understood. Immune checkpoints are essential in preserving self-tolerance and regular immune responses. B, T, and natural killer T cells, together with stimulated dendritic cells and monocytes, all exhibit increased PD-1 expression after activation.26 PD-L1 is located on the membrane of cancer cells.27 Blocking of anti-tumor immune reactions is the outcome of the PD-1/PD-L1 interaction.28 As previously mentioned, CTLA-4 is found on the surface of T cells and competes with CD28 to attach CD 80-86 to the dendritic cells.29
Additionally, in secondary lymphedema, CTLA-4 can control the interactions between T cells and antigen-presenting cells.30 Due to the activation of T cells in cancerous cells caused by the ICI-related inhibition of the PD-1/PD-L1 and CTLA-4 axis, tumor cells could be killed by the release of inflammatory mediators and cytokines. The kickoff of this blockade, however, might cause the liberation of tissue-specific self-reactive T cells that can lower or interrupt immune tolerance against renal antigens. The aberrant activated T cells flow into the kidney, enter the parenchyma, and liberate cytokines that cause an inflammatory response and subsequent renal damage.8,14,31
In an empirical study, PD-1 knockout animals established persistent systemic inflammation and a renal lesion that resembled lupus glomerulonephritis,32 providing proof that PD-1 suppression or deletion can have negative effects on the kidney. Moreover, exposure to ICIs leads to the generation of circulating antibodies against native antigens or drug-specific antibodies against renal tissue as a result of altering the immune microenvironment.33 This could result in AIN linked to immune complexes. The secondary activation of drug-specific T cells by ICI exposure might plummet the tolerance of these cells to AIN-related drugs. Patients receiving combination therapy of ICIs and PPIs or NSAIDs may be more likely to experience nephrotoxicity as a result of the decreased tolerance. Another proposed mechanism for ICI-related AKI is haptenization.
Tubule cells of the kidney, mesangial cells, or podocytes may identify small-molecular-weight pharmacological molecules such as ICIs or their metabolized constituents as an antigen, resulting in the production of an antigen-antibody complex.34 Moreover, dendritic cells in the parenchyma of the kidney can capture and identify ICIs as a hapten, resulting in tubular destruction and kidney disease.35
Immune-related Adverse Effects
Typically, immune checkpoints decrease the expression of T cell responses to protect against probable destructive immune responses, including autoimmune disease. Nevertheless, cancer cells can hijack this pathway to escape the immune system by triggering checkpoints and prohibition of the T cells. Therefore, cancer patients may benefit from treatment when these immune checkpoint pathways are disrupted as this can trigger an anti-tumor immune response. The types of irAEs associated with single-drug treatment that target the CTLA-4 or PD-1 cascades vary, which is in line with the unique roles of immune checkpoints.36 PD-1 and PD-L1 blockers are usually better tolerated than the inhibitors of CTLA-4. A systematic review found that irAEs of grade > 3 are more frequent with CTLA-4 blockers.37,38 It is unclear exactly what biological processes underlie the variations in irAE severity and location with various ICIs. CTLA-4 blockade may result in more T cell growth or less CD4 + CD25 + regulatory T (Treg) cell-related suppression of the immune system, while PD-1 inhibition will likely stimulate fewer T cell clones.39,40 Furthermore, PD-1 can be produced in circulating T cells upon activation during T cell receptor-based signaling even though the majority of them do not express it.39
In general, the types of irAEs do not appear to be cancer-specific.37 While cross-study evaluations must be interpreted cautiously, certain information indicates that distinct irAE patterns may be driven by distinct organ-specific immune microenvironments in certain cancers as the occurrence of particular irAEs differs amongst different cancer patients receiving identical ICI. Comparing irAEs caused by PD-1 blockers in renal cell carcinoma, non-small-cell lung cancer, and melanoma, for instance, revealed that melanoma cases experienced a lower occurrence of pneumonitis but a higher incidence of gastrointestinal and skin-related irAEs compared with patients with other cancers.38
As part of their normal function to preserve self-tolerance, Treg cells express CTLA-4 and suppress the immune system by preventing effector T cell growth and the release of cytokine. Anti-CTLA-4 antibody administration reduces Treg cell survival and function in mice through antibody-dependent cell-related cytotoxicity. This raises the T cells/ Treg cells ratio in the tumor microenvironment, potentially boosting anti-tumor responses and adding to the beneficial effects of CTLA-4 inhibitors.41 These in vivo results indicated that ipilimumab-treated patients have fewer circulating Treg cells overall, but their relative rates of naive, central memory, and effector memory cells remain unchanged.42 Unbalanced Treg and sort 17 T helper (TH17) cells may be an influencer in irAEs connected with ICIs although this decrease was not supported by all studies.43,44 Several autoimmune diseases are closely linked to enhanced TH17 cell responses because these cells produce proinflammatory cytokines.45 Moreover, augmented IL-17 amount was linked to irAEs with high severity, specifically colitis, in ipilimumab-treated patients, indicating that the impact of ICIs on TH17 cells is related to the exhibition of irAEs. It is noteworthy that CTLA-4 inhibitors upsurge the number of circulating TH17 cells in melanoma patients, particularly in irAEs-developed cases.46,47
Management of Nephrotoxicity
Until proven otherwise, a substantial rise in Cr levels must be taken as a sign of immune-related nephritis during ICI therapy. Due to the degree of toxicity, complication management and starting prompt therapy are strongly advised after the most frequent causes of AKI have been ruled out. As irAEs occur less commonly, no controlled clinical trial has previously been designed to specifically assess the results of therapeutic management and recommendations.48-50
Discontinuation of ICIs and administration of corticosteroids in acute tubulointerstitial nephritis are recommended for first-line therapy.13,51,52 Nevertheless, according to recent research, prednisone in high doses (1 mg/kg at least) should only be used until baseline kidney function restoration or for a maximum of three weeks before a 5- to 6-week tapering period.53 Furthermore, a retrospective study found that the risk of recurrence and recovery of kidney function was comparable for patients with ICI-associated AKI receiving corticosteroids for shorter periods (28 days or less) compared to longer periods.54 Accordingly, the ASCO guidelines recommend using additional immunosuppressive drugs such as infliximab, azathioprine, cyclosporine, cyclophosphamide, or mycophenolate if steroid therapy is ineffective.55
According to retrospective research and case reports, Mycophenolate 1 g twice a day was effective in treating individuals with steroid-refractory irAEs, particularly kidney-related ones.56 The ASCO recommendations state that all patients who experience grade 3 AKI should have their ICIs permanently stopped even though doing so may prevent them from receiving a treatment that could save their lives. Hence, consideration should be given to prior outcomes as well as any available alternative therapies. Rechallenging ICI seems to be a viable and active strategy, but more research is needed to determine whether it is safe to do so after ICI-AKI before considering ICIs once renal injury has been resolved or stopped.
It seems that a multidisciplinary approach is necessary for oncologic patient management and nephrologist cooperation for the prevention and management of nephrotoxicity, and developing guidelines is required for anticancer therapies.57
Pharmacokinetics of Immune Checkpoint Inhibitors
Since all ICIs have just recently received approval, there is not much information on the pharmacokinetics/pharmacodynamics relationship between ICIs. The majority of our understanding stems from registration trials conducted in particular patient cohorts. Upon the approval of initial mAbs in cancer therapy, years of clinical experience have been required to comprehend that PK factors may be just as important as somatic biomarkers in predicting clinical consequences.58,59 An understanding of exposure-response associations and the way available biomarkers can predict or anticipate them can help treatment optimization and patient selection for ICIs. Furthermore, characterizing the causes of variation amongst ICIs and evaluating the significance of various substitute endpoints in clinical trials may contribute to the advancement of new drug development.60
ICIs are dispersed and metabolized through a variety of pathways following intravenous administration. Significant attachment to target antigens in plasma and/or tissues lowers the concentration of free ICIs and expands the distribution volume. The main mechanism controlling the transvascular drive of unbound ICIs is convection, the extent of which is restricted by endothelial permeability and organ perfusion. Diffusion and convection show how ICIs are dispersed throughout tissues. By inhibiting the intracellular breakdown of these medications and extending their half-life, the FcRn is in charge of transporting ICIs back into the vascular system. Conversely, clearance increases when antibodies are produced against ICIs. Notwithstanding, the predominant method of ICI clearance persists through proteolytic catabolism, which takes place in peripheral tissues as well as plasma. Finally, an extra clearance pathway is triggered by endocytosis mediated by receptors.61
Conclusion
Despite being a rare side effect of ICI drugs, AKI, failure and delay in diagnosis could result in potentially fatal situations. AKI may develop sometime after the initiation of ICI therapy with a delay of weeks or months, depending on the ICI type. It is advised to stop using ICIs and/or to start using corticosteroids in individuals who have experienced severe toxicity (grade 2). The absence of sensitive or specific clinical signs to precisely identify ICI-AKI in the absence of a renal biopsy necessitates the creation of non-invasive biomarkers (e.g., blood, urinary, and even imaging-dependent biomarkers) to diagnose cases that can be safely retested following a prescription of ICIAKI. Moreover, it is important to note that serious electrolyte imbalances can develop during ICI therapy which require frequent monitoring.
Ethics statement
Not applicable.
Disclosure of funding source
The present study was sponsored by the Kidney Research Center, Tabriz University of Medical Sciences (Grant no: 69392), and the ethical approval code for this project is IR.TBZMED.REC.1401.117.
Conflict of interests declaration
None to be declared.
Acknowledgments
We would like to appreciate the cooperation of the Clinical Research Development Unit of Imam Reza General Hospital, Tabriz, Iran in conducting this research.
Data availability statement
This article contains all data produced or analyzed during this study.
Author contributions
Conceptualization: Elham Ahmadian.
Data curation: Fariba Mahmoodpoor.
Formal analysis: Elham Ahmadian.
Funding acquisition: Elham Ahmadian.
Investigation: Aygun Nasibova.
Methodology: Mahbube Valiyeva.
Project administration: Fariba Mahmoodpoor.
Resources: Elham Ahmadian.
Software: Aygun Nasibova.
Supervision: Elham ahmadian.
Validation: Elham Ahmadian.
Visualization: Fariba Mahmoodpoor.
Writing–original draft: Fariba Mahmoodpoor, Aygun Nasibova, MAhbube Valiyeva.
Writing–review & editing: Elham Ahmadian.
References
- de Miguel M, Calvo E. Clinical challenges of immune checkpoint inhibitors. Cancer Cell 2020; 38(3):326-33. doi: 10.1016/j.ccell.2020.07.004 [Crossref] [ Google Scholar]
- Li B, Chan HL, Chen P. Immune checkpoint inhibitors: basics and challenges. Curr Med Chem 2019; 26(17):3009-25. doi: 10.2174/0929867324666170804143706 [Crossref] [ Google Scholar]
- Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer 2018; 118(1):9-16. doi: 10.1038/bjc.2017.434 [Crossref] [ Google Scholar]
- Kosmaczewska A, Ciszak L, Boćko D, Frydecka I. Expression and functional significance of CTLA-4, a negative regulator of T cell activation. Arch Immunol Ther Exp (Warsz) 2001; 49(1):39-46. [ Google Scholar]
- Wintterle S, Schreiner B, Mitsdoerffer M, Schneider D, Chen L, Meyermann R. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res 2003; 63(21):7462-7. [ Google Scholar]
- Sondak VK, Smalley KS, Kudchadkar R, Grippon S, Kirkpatrick P. Ipilimumab. Nat Rev Drug Discov 2011; 10(6):411-2. doi: 10.1038/nrd3463 [Crossref] [ Google Scholar]
- Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015; 372(21):2006-17. doi: 10.1056/NEJMoa1414428 [Crossref] [ Google Scholar]
- Catalano M, Roviello G, Galli IC, Santi R, Nesi G. Immune checkpoint inhibitor induced nephrotoxicity: an ongoing challenge. Front Med (Lausanne) 2022; 9:1014257. doi: 10.3389/fmed.2022.1014257 [Crossref] [ Google Scholar]
- Borghaei H, Smith MR, Campbell KS. Immunotherapy of cancer. Eur J Pharmacol 2009; 625(1-3):41-54. doi: 10.1016/j.ejphar.2009.09.067 [Crossref] [ Google Scholar]
- Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018; 378(2):158-68. doi: 10.1056/NEJMra1703481 [Crossref] [ Google Scholar]
- Franzin R, Netti GS, Spadaccino F, Porta C, Gesualdo L, Stallone G. The use of immune checkpoint inhibitors in oncology and the occurrence of AKI: where do we stand?. Front Immunol 2020; 11:574271. doi: 10.3389/fimmu.2020.574271 [Crossref] [ Google Scholar]
- Wanchoo R, Karam S, Uppal NN, Barta VS, Deray G, Devoe C. Adverse renal effects of immune checkpoint inhibitors: a narrative review. Am J Nephrol 2017; 45(2):160-9. doi: 10.1159/000455014 [Crossref] [ Google Scholar]
- Perazella MA, Shirali AC. Immune checkpoint inhibitor nephrotoxicity: what do we know and what should we do?. Kidney Int 2020; 97(1):62-74. doi: 10.1016/j.kint.2019.07.022 [Crossref] [ Google Scholar]
- Tinawi M, Bastani B. Nephrotoxicity of immune checkpoint inhibitors: acute kidney injury and beyond. Cureus 2020; 12(12):e12204. doi: 10.7759/cureus.12204 [Crossref] [ Google Scholar]
- Seethapathy H, Herrmann SM, Sise ME. Immune checkpoint inhibitors and kidney toxicity: advances in diagnosis and management. Kidney Med 2021; 3(6):1074-81. doi: 10.1016/j.xkme.2021.08.008 [Crossref] [ Google Scholar]
- Abdel-Rahman O, Fouad M. A network meta-analysis of the risk of immune-related renal toxicity in cancer patients treated with immune checkpoint inhibitors. Immunotherapy 2016; 8(5):665-74. doi: 10.2217/imt-2015-0020 [Crossref] [ Google Scholar]
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012; 120(4):c179-84. doi: 10.1159/000339789 [Crossref] [ Google Scholar]
- Cortazar FB, Marrone KA, Troxell ML, Ralto KM, Hoenig MP, Brahmer JR. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 2016; 90(3):638-47. doi: 10.1016/j.kint.2016.04.008 [Crossref] [ Google Scholar]
- Iacovelli R, Ciccarese C, Fantinel E, Bimbatti D, Romano M, Porcaro AB. Renal toxicity in patients treated with anti-Pd-1 targeted agents for solid tumors. J Onco-Nephrol 2017; 1(2):132-42. doi: 10.5301/jo-n.5000019 [Crossref] [ Google Scholar]
- Gupta S, Short SAP, Sise ME, Prosek JM, Madhavan SM, Soler MJ. Acute kidney injury in patients treated with immune checkpoint inhibitors. J Immunother Cancer 2021; 9(10):e003467. doi: 10.1136/jitc-2021-003467 [Crossref] [ Google Scholar]
- Gupta S, Cortazar FB, Riella LV, Leaf DE. Immune checkpoint inhibitor nephrotoxicity: update 2020. Kidney360 2020; 1(2):130-40. doi: 10.34067/kid.0000852019 [Crossref] [ Google Scholar]
- Trisal SR, Low G, Pathan F, Gangadharan Komala M. Kidney adverse events associated with immune checkpoint inhibitor therapy: a systematic review and Bayesian network meta-analysis. Clin J Am Soc Nephrol 2023; 18(7):843-9. doi: 10.2215/cjn.0000000000000160 [Crossref] [ Google Scholar]
- Tan AJ, Mo DC, Wu K, Pan HM, Wang DM, Xu XX. Nephrotoxicity of immune checkpoint inhibitor combination therapy in patients with advanced renal cell carcinoma: a meta-analysis. World J Urol 2023; 41(6):1563-71. doi: 10.1007/s00345-023-04407-x [Crossref] [ Google Scholar]
- Kanbay M, Copur S, Siriopol D, Yildiz AB, Berkkan M, Popa R. The association between acute kidney injury and outcomes in cancer patients receiving immune checkpoint inhibitor therapy: a systematic review and meta-analysis. Clin Kidney J 2023; 16(5):817-26. doi: 10.1093/ckj/sfac194 [Crossref] [ Google Scholar]
- Panich J, Irwin C, Bissonette A, Elkhidir S, Lodhi F, Folz C, et al. Cohort study on immune checkpoint inhibitor-associated acute kidney injury: incidence, risk factors, and management strategies. J Oncol Pharm Pract. 2023:10781552231171078. 10.1177/10781552231171078.
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008; 26:677-704. doi: 10.1146/annurev.immunol.26.021607.090331 [Crossref] [ Google Scholar]
- Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr Opin Immunol 2007; 19(3):309-14. doi: 10.1016/j.coi.2007.04.012 [Crossref] [ Google Scholar]
- Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res 2020; 10(3):727-42. [ Google Scholar]
- Hosseini A, Gharibi T, Marofi F, Babaloo Z, Baradaran B. CTLA-4: from mechanism to autoimmune therapy. Int Immunopharmacol 2020; 80:106221. doi: 10.1016/j.intimp.2020.106221 [Crossref] [ Google Scholar]
- Lo B, Abdel-Motal UM. Lessons from CTLA-4 deficiency and checkpoint inhibition. Curr Opin Immunol 2017; 49:14-9. doi: 10.1016/j.coi.2017.07.014 [Crossref] [ Google Scholar]
- Mamlouk O, Selamet U, Machado S, Abdelrahim M, Glass WF, Tchakarov A. Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: single-center experience. J Immunother Cancer 2019; 7(1):2. doi: 10.1186/s40425-018-0478-8 [Crossref] [ Google Scholar]
- Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999; 11(2):141-51. doi: 10.1016/s1074-7613(00)80089-8 [Crossref] [ Google Scholar]
- Tucci M, Passarelli A, Todisco A, Mannavola F, Stucci LS, D’Oronzo S. The mechanisms of acute interstitial nephritis in the era of immune checkpoint inhibitors in melanoma. Ther Adv Med Oncol 2019; 11:1758835919875549. doi: 10.1177/1758835919875549 [Crossref] [ Google Scholar]
- Izzedine H, Gueutin V, Gharbi C, Mateus C, Robert C, Routier E. Kidney injuries related to ipilimumab. Invest New Drugs 2014; 32(4):769-73. doi: 10.1007/s10637-014-0092-7 [Crossref] [ Google Scholar]
- Hofmann L, Forschner A, Loquai C, Goldinger SM, Zimmer L, Ugurel S. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer 2016; 60:190-209. doi: 10.1016/j.ejca.2016.02.025 [Crossref] [ Google Scholar]
- Pauken KE, Dougan M, Rose NR, Lichtman AH, Sharpe AH. Adverse events following cancer immunotherapy: obstacles and opportunities. Trends Immunol 2019; 40(6):511-23. doi: 10.1016/j.it.2019.04.002 [Crossref] [ Google Scholar]
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373(1):23-34. doi: 10.1056/NEJMoa1504030 [Crossref] [ Google Scholar]
- Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol 2017; 28(10):2377-85. doi: 10.1093/annonc/mdx286 [Crossref] [ Google Scholar]
- Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol 2018; 8:86. doi: 10.3389/fonc.2018.00086 [Crossref] [ Google Scholar]
- Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017; 377(14):1345-56. doi: 10.1056/NEJMoa1709684 [Crossref] [ Google Scholar]
- Selby MJ, Engelhardt JJ, Quigley M, Henning KA, Chen T, Srinivasan M. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res 2013; 1(1):32-42. doi: 10.1158/2326-6066.cir-13-0013 [Crossref] [ Google Scholar]
- Pico de Coaña Y, Poschke I, Gentilcore G, Mao Y, Nyström M, Hansson J. Ipilimumab treatment results in an early decrease in the frequency of circulating granulocytic myeloid-derived suppressor cells as well as their Arginase1 production. Cancer Immunol Res 2013; 1(3):158-62. doi: 10.1158/2326-6066.cir-13-0016 [Crossref] [ Google Scholar]
- Sharma A, Subudhi SK, Blando J, Scutti J, Vence L, Wargo J. Anti-CTLA-4 immunotherapy does not deplete FOXP3 + regulatory T cells (Tregs) in human cancers. Clin Cancer Res 2019; 25(4):1233-8. doi: 10.1158/1078-0432.ccr-18-0762 [Crossref] [ Google Scholar]
- Knochelmann HM, Dwyer CJ, Bailey SR, Amaya SM, Elston DM, Mazza-McCrann JM. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell Mol Immunol 2018; 15(5):458-69. doi: 10.1038/s41423-018-0004-4 [Crossref] [ Google Scholar]
- Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev 2014; 13(6):668-77. doi: 10.1016/j.autrev.2013.12.004 [Crossref] [ Google Scholar]
- von Euw E, Chodon T, Attar N, Jalil J, Koya RC, Comin-Anduix B. CTLA4 blockade increases Th17 cells in patients with metastatic melanoma. J Transl Med 2009; 7:35. doi: 10.1186/1479-5876-7-35 [Crossref] [ Google Scholar]
- Tarhini AA, Zahoor H, Lin Y, Malhotra U, Sander C, Butterfield LH. Baseline circulating IL-17 predicts toxicity while TGF-β1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J Immunother Cancer 2015; 3:39. doi: 10.1186/s40425-015-0081-1 [Crossref] [ Google Scholar]
- Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018; 36(17):1714-68. doi: 10.1200/jco.2017.77.6385 [Crossref] [ Google Scholar]
- Simonaggio A, Michot JM, Voisin AL, Le Pavec J, Collins M, Lallart A. Evaluation of readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol 2019; 5(9):1310-7. doi: 10.1001/jamaoncol.2019.1022 [Crossref] [ Google Scholar]
- Majem M, García-Martínez E, Martinez M, Muñoz-Couselo E, Rodriguez-Abreu D, Alvarez R. SEOM clinical guideline for the management of immune-related adverse events in patients treated with immune checkpoint inhibitors (2019). Clin Transl Oncol 2020; 22(2):213-22. doi: 10.1007/s12094-019-02273-x [Crossref] [ Google Scholar]
- Moss EM, Perazella MA. The role of kidney biopsy in immune checkpoint inhibitor nephrotoxicity. Front Med (Lausanne) 2022; 9:964335. doi: 10.3389/fmed.2022.964335 [Crossref] [ Google Scholar]
- Seethapathy H, Herrmann SM, Rashidi A. Immune checkpoint inhibitor-associated AKI: debates in diagnosis, management, and rechallenge. Semin Nephrol 2022; 42(6):151346. doi: 10.1016/j.semnephrol.2023.151346 [Crossref] [ Google Scholar]
- Fernandez-Juarez G, Perez JV, Caravaca-Fontán F, Quintana L, Shabaka A, Rodriguez E. Duration of treatment with corticosteroids and recovery of kidney function in acute interstitial nephritis. Clin J Am Soc Nephrol 2018; 13(12):1851-8. doi: 10.2215/cjn.01390118 [Crossref] [ Google Scholar]
- Gupta S, Garcia-Carro C, Prosek JM, Glezerman I, Herrmann SM, Garcia P. Shorter versus longer corticosteroid duration and recurrent immune checkpoint inhibitor-associated AKI. J Immunother Cancer 2022; 10(9):e005646. doi: 10.1136/jitc-2022-005646 [Crossref] [ Google Scholar]
- Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol 2021; 39(36):4073-126. doi: 10.1200/jco.21.01440 [Crossref] [ Google Scholar]
- Daanen RA, Maas RJH, Koornstra RHT, Steenbergen EJ, van Herpen CML, Willemsen A. Nivolumab-associated nephrotic syndrome in a patient with renal cell carcinoma: a case report. J Immunother 2017; 40(9):345-8. doi: 10.1097/cji.0000000000000189 [Crossref] [ Google Scholar]
- Longhitano E, Muscolino P, Lo Re C, Ferrara SA, Cernaro V, Gembillo G. Immune checkpoint inhibitors and the kidney: a focus on diagnosis and management for personalised medicine. Cancers (Basel) 2023; 15(6):1891. doi: 10.3390/cancers15061891 [Crossref] [ Google Scholar]
- Pointreau Y, Azzopardi N, Ternant D, Calais G, Paintaud G. Cetuximab pharmacokinetics influences overall survival in patients with head and neck cancer. Ther Drug Monit 2016; 38(5):567-72. doi: 10.1097/ftd.0000000000000321 [Crossref] [ Google Scholar]
- Caulet M, Lecomte T, Bouché O, Rollin J, Gouilleux-Gruart V, Azzopardi N. Bevacizumab pharmacokinetics influence overall and progression-free survival in metastatic colorectal cancer patients. Clin Pharmacokinet 2016; 55(11):1381-94. doi: 10.1007/s40262-016-0406-3 [Crossref] [ Google Scholar]
- Feng Y, Masson E, Dai D, Parker SM, Berman D, Roy A. Model-based clinical pharmacology profiling of ipilimumab in patients with advanced melanoma. Br J Clin Pharmacol 2014; 78(1):106-17. doi: 10.1111/bcp.12323 [Crossref] [ Google Scholar]
- Centanni M, Moes D, Trocóniz IF, Ciccolini J, van Hasselt JGC. Clinical pharmacokinetics and pharmacodynamics of immune checkpoint inhibitors. Clin Pharmacokinet 2019; 58(7):835-57. doi: 10.1007/s40262-019-00748-2 [Crossref] [ Google Scholar]