Int J Drug Res Clin. 2023;1:e23.
doi: 10.34172/ijdrc.2023.e23
Original Article
Crocin Protects Against Atrazine-induced Toxicity in Primary Rat Hepatocytes
Mohammad Ali Eghbal 1  , Fariba Mahmoodpoor 2
, Fariba Mahmoodpoor 2  , Elham Ahmadian 3, *
, Elham Ahmadian 3, *  , Sara Aghdasi 4
, Sara Aghdasi 4
Author information:
1Department of Pharmacology and Toxicology, Faculty of Pharmacy, Tabriz University of medical sciences, Tabriz, Iran
2Research Center for Integrative Medicine in Aging, Aging Research Institute,Tabriz University of Medical Sciences, Tabriz, Iran
3Kidney Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
4Graduated from Faculty of Veterinary Medicine, Urmia University, Urmia, Iran
Abstract
Background:
Atrazine is a typical herbicide that has domestic and agricultural uses all over the world. A review of the literature, however, revealed evidence of its destructive effects on many human tissues and signaling networks. As such, this study explored the impact of crocin, a natural pigment, on reducing the liver damage caused by atrazine in primary rat hepatocytes.
Methods:
As biochemical cytotoxicity indicators, cell death, lactate dehydrogenase (LDH) leakage, reactive oxygen species (ROS) generation, lipid peroxidation (LPO), glutathione (GSH) level, and mitochondrial membrane potential were assessed.
Results:
In the first step, LD50 concentration of atrazine was evaluated using a methyl thiazolyl tetrazolium (MTT) test in rat hepatocytes. The findings indicated that cellular function declines at LC50 concentration (400 M). On the contrary, crocin (50 µM) substantially boosted hepatocyte viability, decreased ROS production and LPO, replenished cellular GSH pools, and improved mitochondrial function.
Conclusion:
Overall, the data suggest that crocin may play a protective function in atrazine-induced liver injury in which the main mechanisms of toxicity appear to be the generation of ROS and mitochondrial damage.
Keywords: Crocin, Saffron, Oxidative stress, Hepatotoxicity
Copyright and License Information
© 2023 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
In agricultural settings, atrazine (ATZ) or 2-chloro-4-ethylamino-6-isopropylamino-s-triazine is one of the most widely applied herbicides.1 This herbicide is typically applied in sprays, liquids, concentrates, or granules. ATZ exerts minimal biodegradation in soil and water, which is most often found as a pesticide in surface waterways, so it influences the environment extensively.2
Most body organs are affected by ATZ; furthermore, its impacts on the neurological, excretory, and reproductive systems have been thoroughly studied. ATZ is an endocrine disruptive pollutant that can cause thyroid cancer in female spouses of pesticide applicators in agricultural cohorts.3,4 alter the reproductive system of male rats, and deplete testis and epididymis antioxidant reservoirs. The liver exhibits signs of ATZ poisoning. The submandibular salivary glands of rats given oral ATZ exhibited oxidative stress, degeneration, and apoptosis.5 Rats given ATZ exhibited damaged hepatocytes, substantially elevated levels of total bilirubin, alanine aminotransferase, and aspartate aminotransferase, and decreased glutathione (GSH).6 Rats treated with even low dosages of ATZ exhibited lipidosis in hepatocytes, hepatic peri-acinar necrosis, and portal lymphocytic inflammation.6,7 Genotoxicity has also been observed in the liver after subacute exposure to ATZ.8 Since ATZ is a commonly used herbicide, cases of poisoning are also common. As the liver is the primary organ of chemical exposure, there is no surprise that hepatotoxicity frequently occurs. Therefore, the introduction of novel therapeutic agents to prevent herbicide-induced hepatotoxicity is an urgent issue.
Saffron (Crocus sativus L.) is employed in complementary medicine as a natural therapy against human diseases because it has antioxidant, anti-inflammatory, and anti-carcinogenic characteristics in addition to being hypolipidemic.9,10 Crocin, crocetin, and safranal are the three primary components of saffron. Crocin exhibits strong antioxidant activity and scavenges free radicals.11
One of the most effective strategies for combating hepatotoxicity in recent years has been the phytomedicine approach. The free radical scavenging functions of phytochemicals are mainly responsible for this protection. Therefore, the chief objective of the present investigation was to demonstrate the protective role of crocin against ATZ-associated liver injury.
Methods
Chemicals
Fetal bovine serum and Dulbecco’s Modified Eagle medium (DMEM) were obtained from Invitrogen Gibco. Albumin bovine serum was purchased from the Roche diagnostic company (IN). Collagenase (Type II), thiobarbituric acid, and all other chemicals were acquired from Sigma-Aldrich Co. (Heidelberg, Germany).
Primary Rat Hepatocytes Culture
Collagenase perfusion was used to produce primary rat hepatocytes,12 and the Trypan blue exclusion testing was implemented to assess the viability of the cells to obtain over 85% of viable cells for subsequent investigations. The separated hepatocytes were then incubated for 3 hours at 37 °C in a humidified atmosphere of 95% O2 and 5% CO2 and supplemented with 10% fetal bovine serum and 1% antibiotic-antimycotic solution. Hepatocytes were seeded in 12 well plates (2 × 105/mL) for various experiments and in 96 well plates (4.8 × 104/mL) for the viability tests. Crocin was administered one hour after the cells had been exposed to ATZ for 4 hours, and the cells were then incubated for another 3 hours at 37 °C. In the end, cultured hepatocytes were used to test cytotoxicity and other biochemical tests, including membrane injuries such as lactate dehydrogenase (LDH) leakiness, reactive oxygen species (ROS) production, mitochondrial membrane potential (MMP), GSH level, and lipid peroxidation (LPO).
Cell Viability
Cell viability was assessed using methyl thiazolyl tetrazolium (MTT) and LDH activity. After incubating cells in 96-well plates, 50 mL of medium from every group was exposed to freshly made -NADH solution and left for 20 minutes for LDH measurement. At the same time, an enzyme-linked immunosorbent assay microplate reader was applied to quantify the reaction mixture’s absorbance at 340 nm. The leftover medium was subjected to the MTT test by being incubated by MTT (1 mg/mL) for 4 hours at 37 °C. After the medium was picked up, the produced formazan crystals were dissolved in dimethyl sulfoxide. Finally, using an enzyme-linked immunosorbent assay microplate reader, the absorbance was read at 570 nm, and the consequent data were represented as a percentage of the non-treated group.
Preparing of Cell Lysate
Hepatocytes were exposed to a centrifugation step (5000 g, 5 minutes) before washing with ice-cold phosphate-buffered saline (PBS) to create the cell lysate. Afterward, the cell pellet was reconstituted with PBS and the resultant was sonicated three times for 30 seconds each time using an ultrasonicator (Parasonic 30S, Iran). Furthermore, Lowry’s technique was applied to estimate the amount of total protein.13
Reactive Oxygen Species
Using the fluorescent probe 2′, 7′-dichlorodihydrofluorescein diacetate (DCFH-DA), oxidative stress was studied. This dye can penetrate the cells. Rat hepatocytes were treated and then exposed to the dye solution. Hepatocytes were then incubated for 1 hour at 37 °C. DCFH-DA is broken down by intracellular esterases to DCFH, which can then react with different types of ROS to produce the extremely fluorescent substance known as dichlorofluorescein (DCF). The amount of DCF was determined from a DCF standard via a spectrophotometer with an excitation and emission wavelengths of 485 and 535 nm, correspondingly.
Lipid Peroxidation
In sum, a solution of combined potassium chloride and ferric chloride was added to 0.5 mL of the cell lysate for 30 minutes at 37 °C. The reaction was broken off by adding 2.0 mL of an ice-cold solution of hydrochloric acid, thiobarbituric acid, trichloroacetic acid, and butylated hydroxytoluene. This solution was re-heated at 90 °C for a further 30 minutes. The mixes were then chilled and centrifuged at 7000 g (5 minutes). Further, the percentage of LPO was used to express the results according to the absorbance of the supernatants.
The Level of Glutathione
The level of GSH in hepatocyte cell lysates was calculated using the method developed by Popet et al.14 To do this, cell lysate (50 µL) was exposed to DTNB reagent [(150 µL) 12 mM (NADPH 12mM, GSH reductase 50 U/mL, DTNB 0.1 mM] and left over for 45 minutes. Then, the results were reported as nmol/mg protein through a calibration curve based on the amounts of GSH.
Mitochondrial Membrane Potential
Hepatocytes were subjected to ATZ and crocin treatment before being incubated for 20 minutes at 37 °C in the dark with a medium encompassing 1.6 M of Rhodamine 123. The method is based on the selective lump of Rhodamine 123 by active mitochondrion. The residual Rhodamine 123 was quantified with a spectrophotometer apparatus at 490 nm excitation and 520 nm emission wavelengths, and the results were reported as nmol/mg protein.
Statistical Analysis
For three separate experiments, the results were presented as the mean ± standard error of the mean. A one-way analysis of variance was utilized for the statistical analysis, followed by Tukey’s post hoc test, and the P value below 0.05 was regarded as statistically significant.
Results
The viability of rat hepatocytes was treated with ATZ in escalating doses to perform the MTT test. Concentration-dependent toxicity was noted, with the ATZ LC50 (the concentration that caused 50% of the hepatocytes to die) being 400 µM, as shown in Figure 1A. Moreover, crocin (50 µM) could significantly reduce the ATZ-induced toxicity, as illustrated in Figure 1B. Lower crocin concentrations, however, did not exhibit any significant protective effect. This study looked into LDH leakage, a crucial indicator of damaged cell membranes. When crocin was added, LDH leakage significantly decreased, indicating protection against ATZ-related membrane damage. Nevertheless, the treatment of hepatocytes with ATZ markedly accelerated LDH leakage (Figure 2).
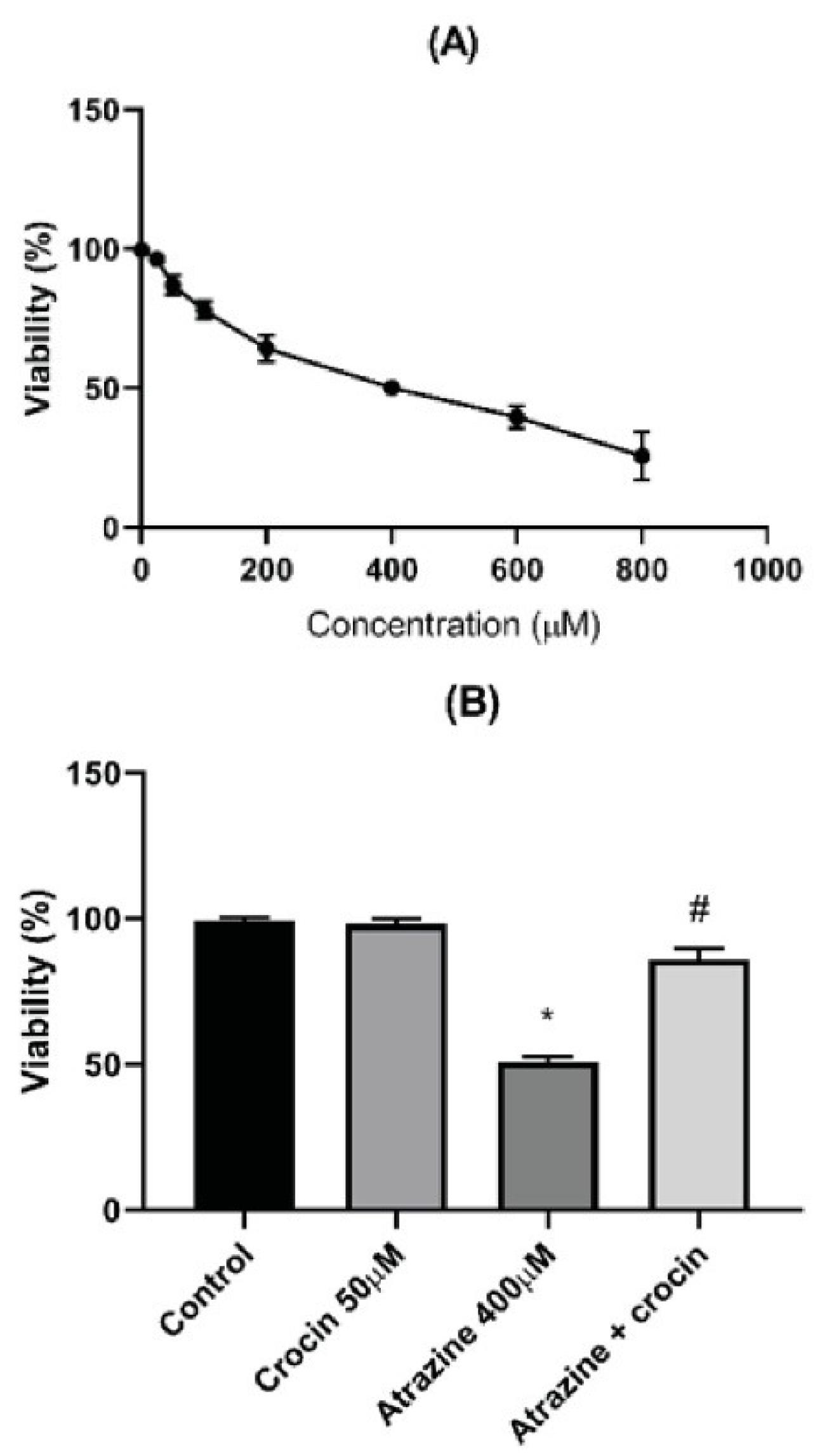
Figure 1.
Viability of Rat Hepatocytes Tested with MTT Assay. (A). Effect of crocin (20 μM) on the viability of ATZ-treated hepatocytes (B). Results are represented as mean ± SD of 3 independent experiments. Note. MTT: Methyl thiazolyl tetrazolium; ATZ: Atrazine; SD: Standard deviation. * Significant compared to non-treated; # Significant in comparison with ATZ–treated hepatocytes (P < 0.05)
.
Viability of Rat Hepatocytes Tested with MTT Assay. (A). Effect of crocin (20 μM) on the viability of ATZ-treated hepatocytes (B). Results are represented as mean ± SD of 3 independent experiments. Note. MTT: Methyl thiazolyl tetrazolium; ATZ: Atrazine; SD: Standard deviation. * Significant compared to non-treated; # Significant in comparison with ATZ–treated hepatocytes (P < 0.05)
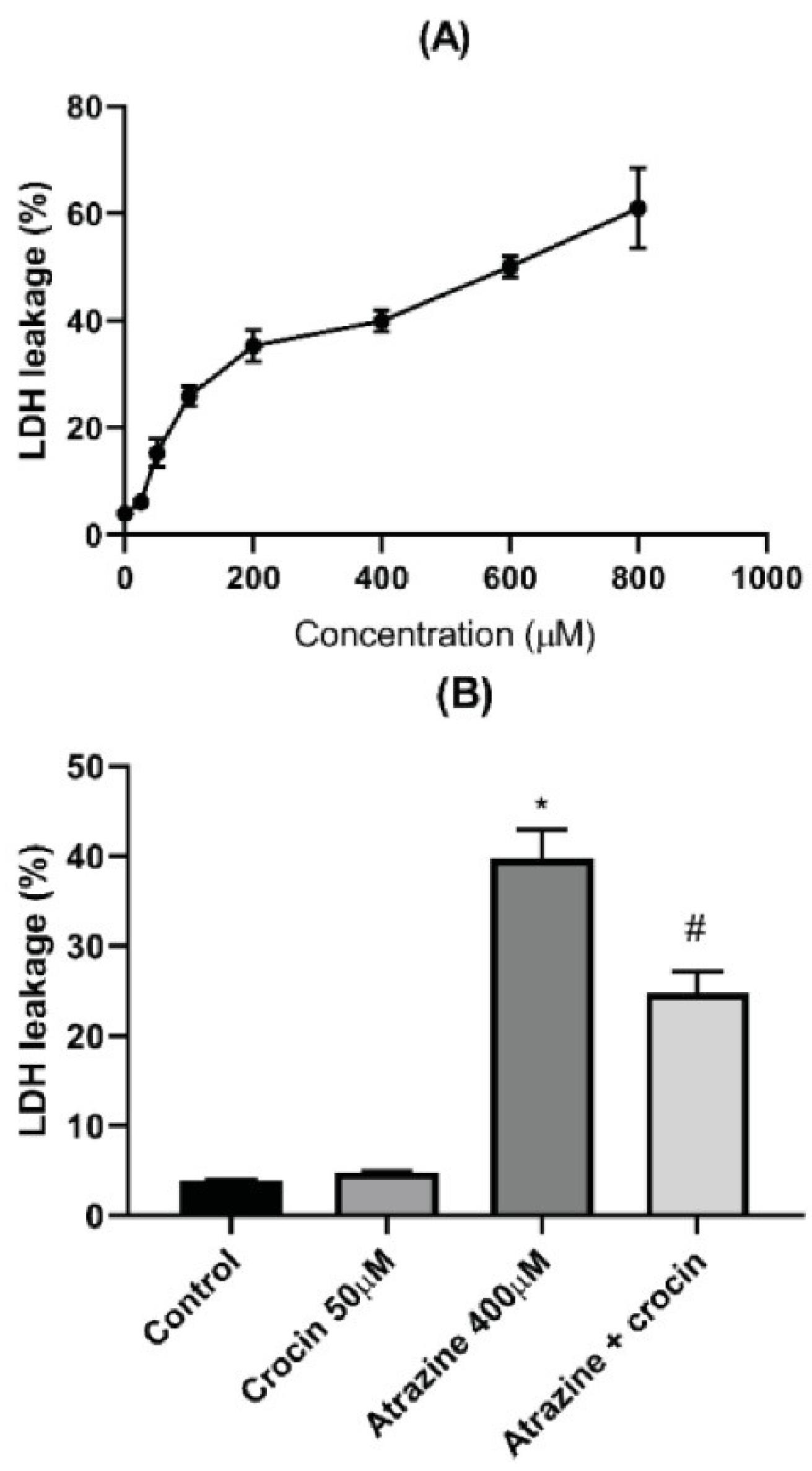
Figure 2.
Membrane Leakage of Rat Hepatocytes Assessed by LDH Test (A).Effect of Crocin (50 μM) on the Membrane Integrity of ATZ-treated Hepatocytes (B). Data are represented as mean±SD of 3 independent experiments. Note. LDH: Lactate dehydrogenase; ATZ: Atrazine. * Significant compared to control; # Significant compared to ATZ–treated hepatocytes (P < 0.05)
.
Membrane Leakage of Rat Hepatocytes Assessed by LDH Test (A).Effect of Crocin (50 μM) on the Membrane Integrity of ATZ-treated Hepatocytes (B). Data are represented as mean±SD of 3 independent experiments. Note. LDH: Lactate dehydrogenase; ATZ: Atrazine. * Significant compared to control; # Significant compared to ATZ–treated hepatocytes (P < 0.05)
Hepatocytes exposed to ATZ exhibited a surge in cellular ROS generation. Moreover, crocin at a 50 µM concentration could significantly reduce DCF fluorescence (Figure 3). Oxidative danger frequently occurs after the breakdown of cellular lipids. Furthermore, the LC50 concentration of ATZ boosted ROS production while also causing the membrane lipids to degrade, and crocin (50 µM) significantly reduced the quantity of LPO (Figure 4).
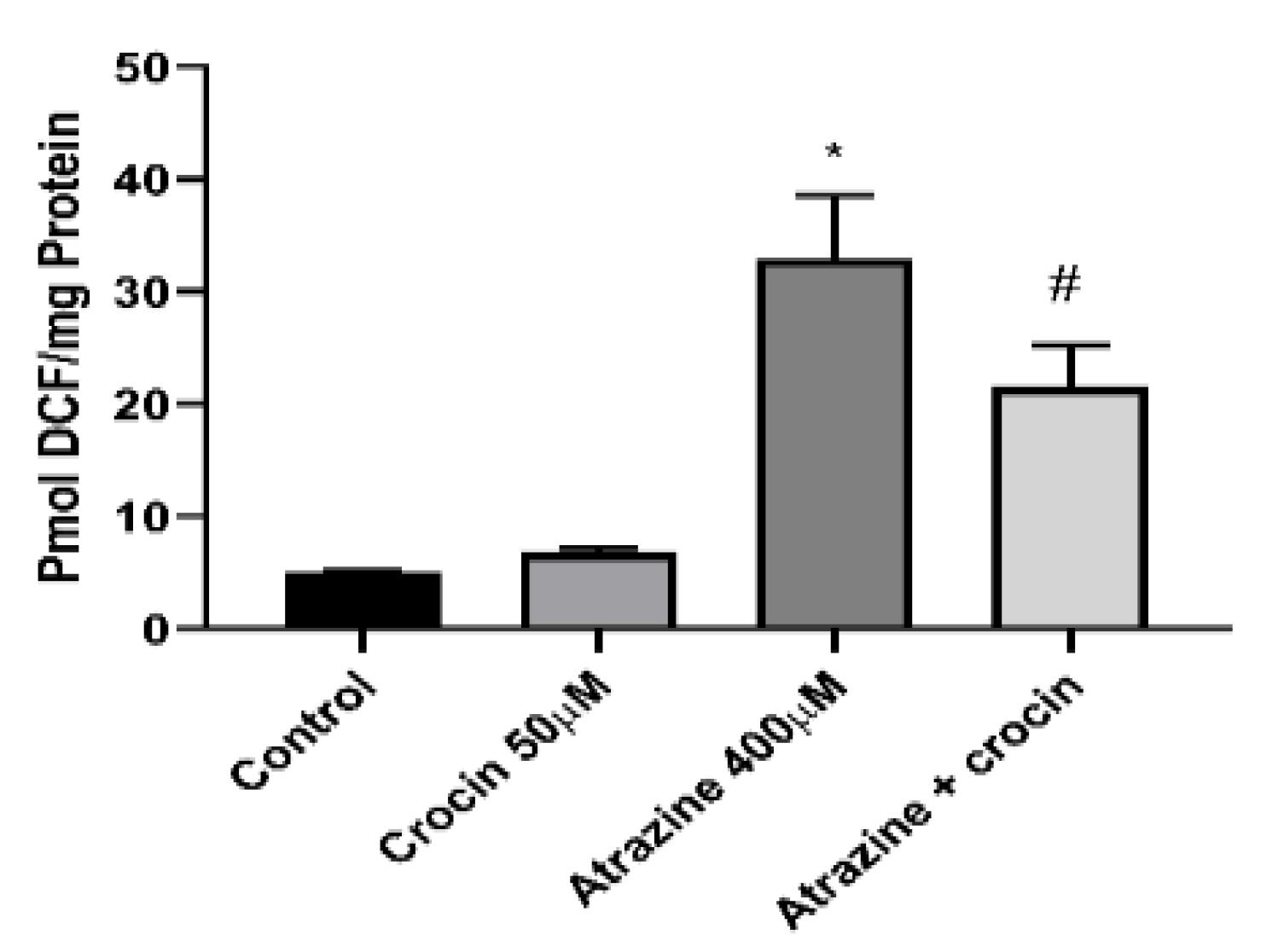
Figure 3.
Effect of Crocin (50 μM) on the ROS Formation of ATZ-Treated Cells. Note. ROS: Reactive oxygen species; ATZ: Atrazine; SD: Standard deviation. Results are represented as mean ± SD of 3 independent experiments. * Significant in comparison with non-treated; # Significant compared ATZ–treated hepatocytes (P < 0.05)
.
Effect of Crocin (50 μM) on the ROS Formation of ATZ-Treated Cells. Note. ROS: Reactive oxygen species; ATZ: Atrazine; SD: Standard deviation. Results are represented as mean ± SD of 3 independent experiments. * Significant in comparison with non-treated; # Significant compared ATZ–treated hepatocytes (P < 0.05)
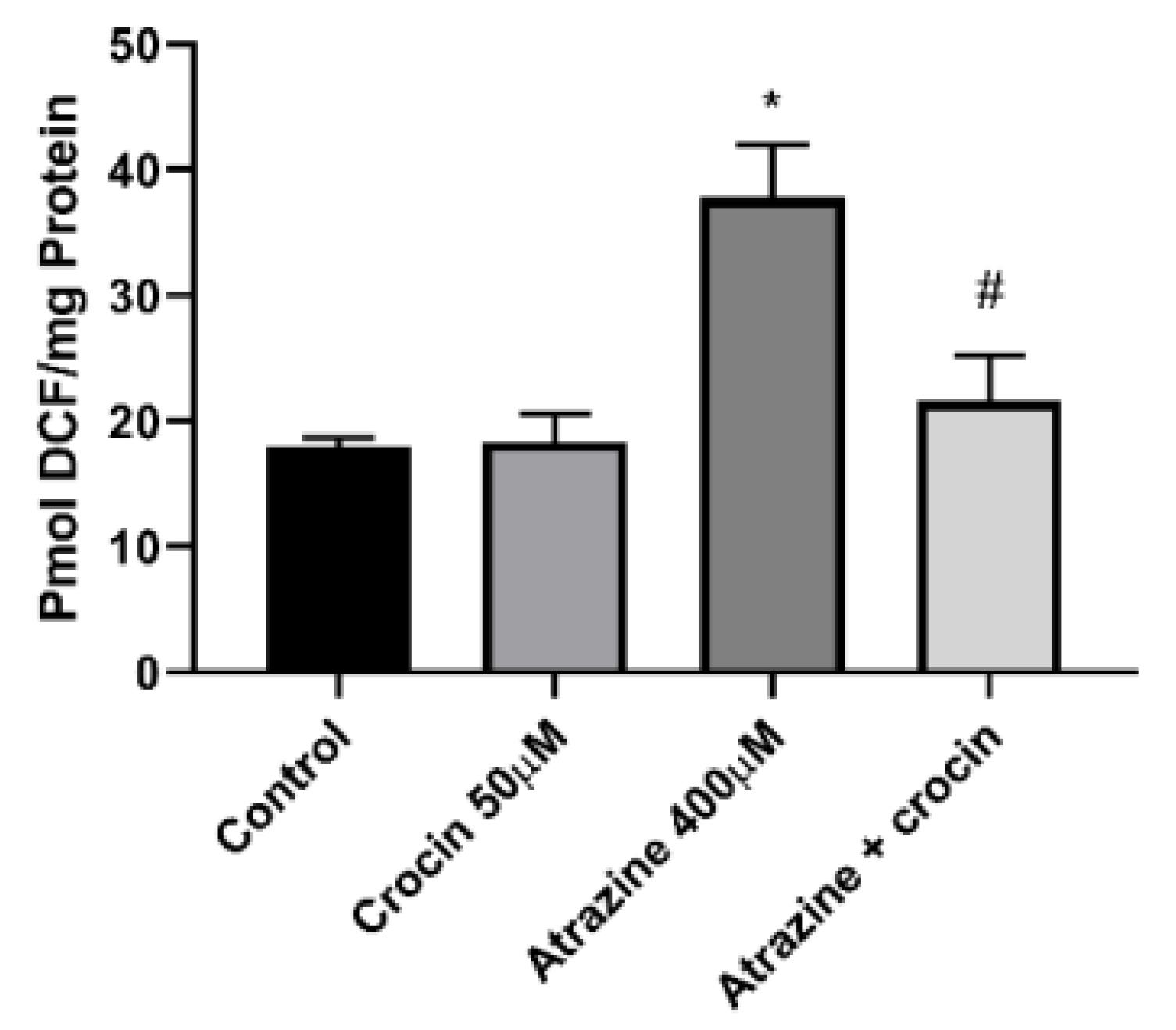
Figure 4.
Effect of Crocin (50 μM) on the LPO of ATZ-Treated Cells. Note. LPO: Lipid peroxidation; ATZ: Atrazine; SD: Standard deviation. Results are represented as mean ± SD of 3 independent experiments. * Significant in comparison with non-treated; # Significant compared with ATZ–treated hepatocytes (P < 0.05)
.
Effect of Crocin (50 μM) on the LPO of ATZ-Treated Cells. Note. LPO: Lipid peroxidation; ATZ: Atrazine; SD: Standard deviation. Results are represented as mean ± SD of 3 independent experiments. * Significant in comparison with non-treated; # Significant compared with ATZ–treated hepatocytes (P < 0.05)
The major endogenous antioxidant protection against xenobiotic-induced toxicities in cells is GSH. ATZ (400 µM) substantially plummeted cellular GSH levels in comparison with the control group, while crocin (50 µM) therapy of hepatocytes effectively refilled cellular GSH reservoirs (Figure 5). Figure 6 depicts the effect of ATZ on the activation of mitochondrial membrane breakdown. ATZ significantly decreased MMP in the culture media when applied to hepatocytes, but crocin (50 µM) exhibited observable effects on reversing mitochondrial activity (Figure 6).
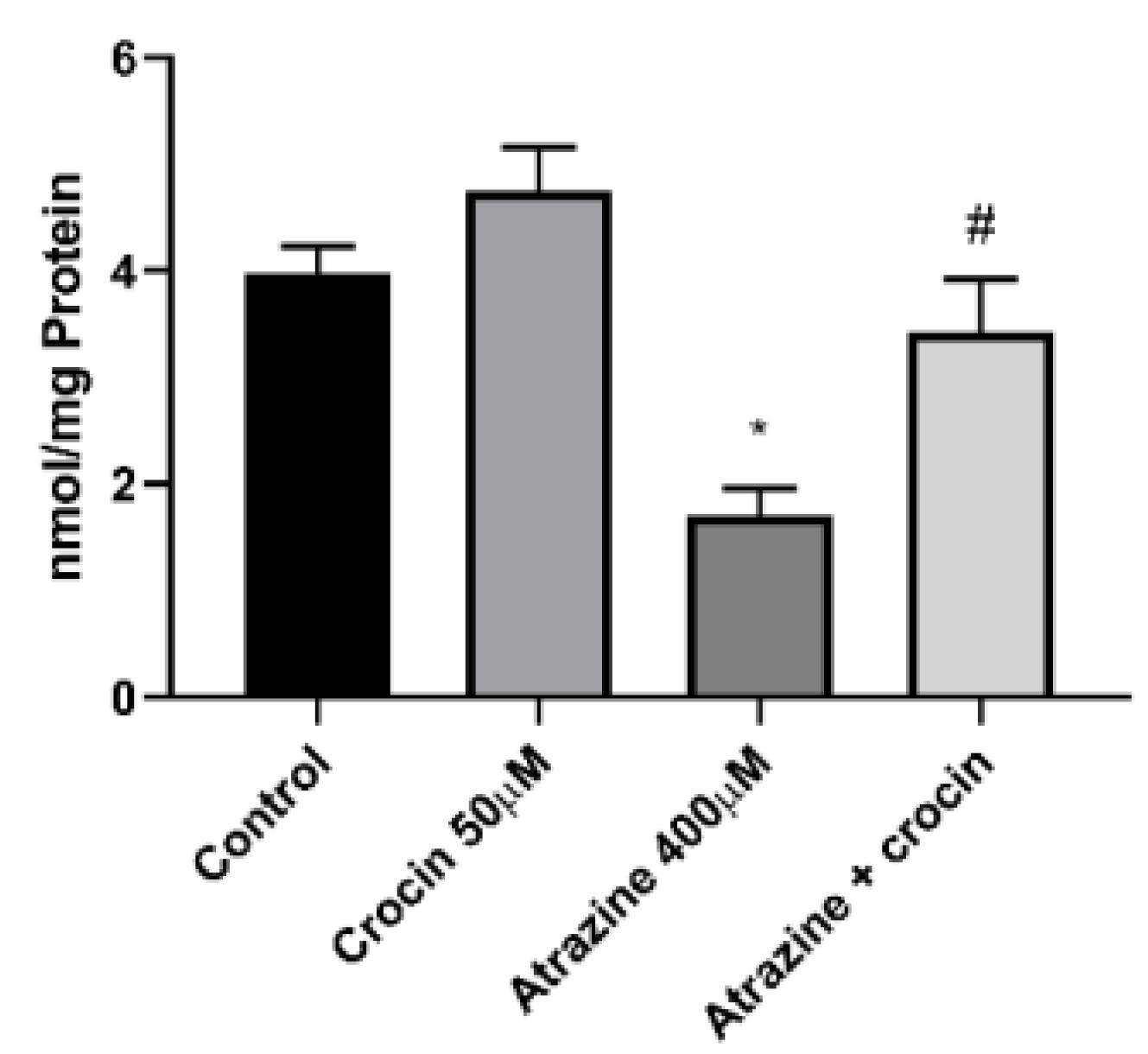
Figure 5.
Effect of Crocin (50 μM) on the Glutathione Level of ATZ-Treated Cells. Note. ATZ: Atrazine; SD: Standard deviation. Results are represented as mean ± SD of 3 independent experiments. * Significant in comparison with non-treated; # Significant compared with ATZ–treated hepatocytes (P < 0.05)
.
Effect of Crocin (50 μM) on the Glutathione Level of ATZ-Treated Cells. Note. ATZ: Atrazine; SD: Standard deviation. Results are represented as mean ± SD of 3 independent experiments. * Significant in comparison with non-treated; # Significant compared with ATZ–treated hepatocytes (P < 0.05)
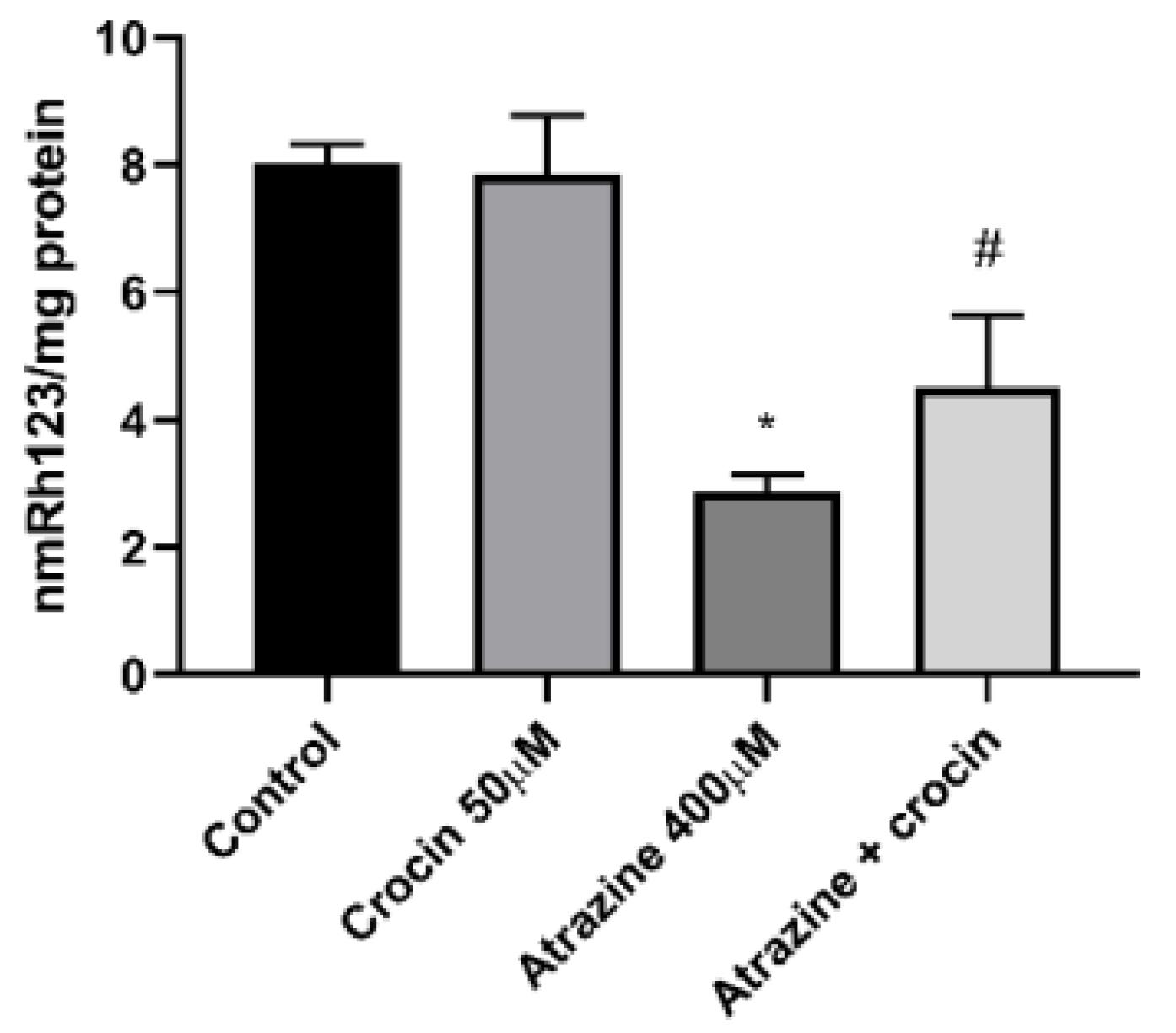
Figure 6.
Effect of Crocin (50 μM) on the MMP of ATZ-Treated Cells. Note. MMP: Mitochondrial membrane potential; ATZ: Atrazine; SD: Standard deviation. Results are represented as mean ± SD of 3 independent experiments. * Significant in comparison with non-treated; # Significant compared with ATZ–treated hepatocytes (P < 0.05)
.
Effect of Crocin (50 μM) on the MMP of ATZ-Treated Cells. Note. MMP: Mitochondrial membrane potential; ATZ: Atrazine; SD: Standard deviation. Results are represented as mean ± SD of 3 independent experiments. * Significant in comparison with non-treated; # Significant compared with ATZ–treated hepatocytes (P < 0.05)
Discussion
Exogenous substances such as xenobiotics and poisons are extensively metabolized in hepatic tissue, producing a variety of metabolites that can either be less harmful or more toxic depending on their structure.15 Moreover, the risk of hepatotoxicity increases because the liver is the primary site of xenobiotic exposure.16,17 The investigation of potential toxicological pathways and the introduction of protective chemicals become crucial correspondingly. The occurrence of oxidative hazard is regarded as one of the main mechanisms of liver damage. ATZ-mediated toxicity has been linked to oxidative damage.18 After intoxication, ROS formation can disrupt the cell membrane.19 Furthermore, ROS-induced changes in cellular constituents might result in the denaturation of proteins, peroxidation of unsaturated fatty acids, and depletion of the cellular antioxidant pool.20 In the present study, LPO production as a sign of oxidative stress following ATZ administration is consistent with earlier research.21 Hence, using antioxidants to combat such toxins may be a beneficial strategy. Natural antioxidants have drawn interest in this regard due to their safer performance. According to a study by Hosseinzadeh et al, crocin effectively reduced ischemia-reperfusion-related oxidative injury in vivo.22 Likewise, according to Mehri and colleagues’ study, crocin reduced the acrylamide-associated neurotoxicity in rats by preventing oxidative stress.23 Moreover, it has been found that the antioxidant capabilities of crocin and saffron extract decrease malondialdehyde levels and ROS-stimulated peroxidation of membrane lipids.24 Saffron and crocin have also been reported to regulate oxidative indicators in the hippocampal function.25 These studies support our findings, reporting that after crocin administration, ROS formation and further LPO were plunged, GSH levels were replenished, and MMP increased. Different studies have revealed the cytotoxic effects of ATZ in which oxidative stress-related pathways play a pivotal role. For instance, ATZ induces oxidative-stress-mediated apoptosis in the testicular tissue of male offspring whose mothers were exposed to this herbicide. This can lead to a plummeted number of sperms, elevated sperm abnormality, and spermatogenesis. It has been reported that the treatment of animals with crocin during pregnancy and lactation significantly reverses ATZ-induced damages.26 Therefore, the use of crocin as a potent antioxidant agent in reducing ATZ-induced hepatotoxicity can be explained. Furthermore, earlier research showed that crocin dramatically decreases malondialdehyde levels in animal models treated with cisplatin, cyclophosphamide, and diazinon while increasing the liver’s GSH content.27-29
According to Lieshout et al, consuming foods high in antioxidants could hasten the binding of toxic materials with GSH and their elimination from circulation.30 Antioxidants might then act in concert to shield crucial tissues against oxidative harm. Intrinsic anti-ROS systems in the body include antioxidant enzymes and non-enzymatic antioxidants such as GSH. Monitoring the quantity of cellular GSH can therefore provide us with information about the liver damage caused by ROS. The current study found that ATZ significantly reduces GSH levels, which is consistent with earlier research.31 It has been demonstrated that crocin can neutralize some free radicals and raise cellular GSH.32
As a well-known target and the source of cellular ROS production, mitochondria are vital subcellular organelles.33,34 Furthermore, increased ROS production may increase the sensitivity of mitochondria, which could lead to mitochondrial dysfunction, uncoupling of oxidative phosphorylation, and eventually membrane damage.35 Cytochrome c and other pro-apoptotic elements will therefore be liberated into the cytoplasm.36,37 This study found that mitochondrial damage plays a role in the pathophysiology of ATZ-related hepatotoxicity. Moreover, the preventive mechanisms of crocin against ATZ-induced liver damage may involve mitochondrial pathways.
Conclusion
In summary, the findings demonstrated that crocin administration has protective effects against ATZ-induced liver damage in primary cultured isolated rat hepatocytes. The primary protective mechanisms of crocin appear to be mediated by its antioxidant and mitochondrial protecting activities, which were demonstrated as the inhibition of LPO, prevention of GSH depletion, and reduction of MMP collapse. Therefore, it might be suggested that using crocin as a potent antioxidant and mitochondrial protective agent can be beneficial against various xenobiotics that cause hepatotoxicity, particularly for ROS-related damage. However, further in vitro and in vivo experiments are required to clarify the protective mechanisms of protection, the possible association of divers signaling cascades, and validation of the doses used in animals before clinical trials, which can also be mentioned as the limitation of our study.
Ethics statement
The ethical approval for this project is IR.TBZMED.VCR.REC.1401.382 funded by Tabriz University of Medical Sciences, Tabriz, Iran.
Disclosure of funding source
The present study was sponsored by the Kidney Research Center, Tabriz University of Medical Sciences (Grant No.: 71066).
Conflict of interests declaration
None to be declared.
Acknowledgments
The authors would like to thank the Clinical Research Development Unit of Imam Reza General Hospital, Tabriz, Iran.
Data availability statement
This published paper includes all data produced and/or analyzed during the current study.
References
- Singh S, Kumar V, Chauhan A, Datta S, Wani AB, Singh N. Toxicity, degradation and analysis of the herbicide atrazine. Environ Chem Lett 2018; 16(1):211-37. doi: 10.1007/s10311-017-0665-8 [Crossref] [ Google Scholar]
- Gammon DW, Aldous CN, Carr WC Jr, Sanborn JR, Pfeifer KF. A risk assessment of atrazine use in California: human health and ecological aspects. Pest Manag Sci 2005; 61(4):331-55. doi: 10.1002/ps.1000 [Crossref] [ Google Scholar]
- Pathak RK, Dikshit AK. Atrazine and human health. Int J Ecosyst 2011; 1(1):14-23. doi: 10.5923/j.ije.20110101.03 [Crossref] [ Google Scholar]
- Rohr JR. The atrazine saga and its importance to the future of toxicology, science, and environmental and human health. Environ Toxicol Chem 2021; 40(6):1544-58. doi: 10.1002/etc.5037 [Crossref] [ Google Scholar]
- Ahmed YH, AbuBakr HO, Ahmad IM, Ahmed ZSO. Histopathological, immunohistochemical, and molecular alterations in brain tissue and submandibular salivary gland of atrazine-induced toxicity in male rats. Environ Sci Pollut Res Int 2022; 29(20):30697-711. doi: 10.1007/s11356-021-18399-x [Crossref] [ Google Scholar]
- Riera J, Matus E, Matus L, Molino J. Toxicity of commercial atrazine in rattus novergicus organs as a function of concentration: histopathological, ultrastructural and hematological evaluation. An Acad Bras Cienc 2022; 94(2):e20201125. doi: 10.1590/0001-3765202220201125 [Crossref] [ Google Scholar]
- Das S, Sakr H, Al-Huseini I, Jetti R, Al-Qasmi S, Sugavasi R. Atrazine toxicity: the possible role of natural products for effective treatment. Plants (Basel) 2023; 12(12):2278. doi: 10.3390/plants12122278 [Crossref] [ Google Scholar]
- Campos-Pereira FD, Oliveira CA, Pigoso AA, Silva-Zacarin EC, Barbieri R, Spatti EF. Early cytotoxic and genotoxic effects of atrazine on Wistar rat liver: a morphological, immunohistochemical, biochemical, and molecular study. Ecotoxicol Environ Saf 2012; 78:170-7. doi: 10.1016/j.ecoenv.2011.11.020 [Crossref] [ Google Scholar]
- Mzabri I, Addi M, Berrichi A. Traditional and modern uses of saffron (Crocus sativus). Cosmetics 2019; 6(4):63. doi: 10.3390/cosmetics6040063 [Crossref] [ Google Scholar]
- Broadhead GK, Chang A, Grigg J, McCluskey P. Efficacy and safety of saffron supplementation: current clinical findings. Crit Rev Food Sci Nutr 2016; 56(16):2767-76. doi: 10.1080/10408398.2013.879467 [Crossref] [ Google Scholar]
- Omidkhoda SF, Hosseinzadeh H. Saffron and its active ingredients against human disorders: a literature review on existing clinical evidence. Iran J Basic Med Sci 2022; 25(8):913-33. doi: 10.22038/ijbms.2022.63378.13985 [Crossref] [ Google Scholar]
- Wang K, Shindoh H, Inoue T, Horii I. Advantages of in vitro cytotoxicity testing by using primary rat hepatocytes in comparison with established cell lines. J Toxicol Sci 2002; 27(3):229-37. doi: 10.2131/jts.27.229 [Crossref] [ Google Scholar]
- Peterson GL. Review of the Folin phenol protein quantitation method of Lowry, Rosebrough, Farr and Randall. Anal Biochem 1979; 100(2):201-20. doi: 10.1016/0003-2697(79)90222-7 [Crossref] [ Google Scholar]
- Ahmadian E, Babaei H, Mohajjel Nayebi A, Eftekhari A, Eghbal MA. Mechanistic approach for toxic effects of bupropion in primary rat hepatocytes. Drug Res (Stuttg) 2017; 67(4):217-22. doi: 10.1055/s-0042-123034 [Crossref] [ Google Scholar]
- Almazroo OA, Miah MK, Venkataramanan R. Drug metabolism in the liver. Clin Liver Dis 2017; 21(1):1-20. doi: 10.1016/j.cld.2016.08.001 [Crossref] [ Google Scholar]
- Remmer H. The role of theliver in drug metabolism. Am J Med 1970; 49:617-29. doi: 10.1016/s0002-9343(70)80129-2 [Crossref] [ Google Scholar]
- Lerapetritou MG, Georgopoulos PG, Roth CM, Androulakis LP. Tissue-level modeling of xenobiotic metabolism in liver: an emerging tool for enabling clinical translational research. Clin Transl Sci 2009; 2(3):228-37. doi: 10.1111/j.1752-8062.2009.00092.x [Crossref] [ Google Scholar]
- Rashad WA, Saadawy SF, Refaay NE. Mitigating effect of L-carnitine against atrazine-induced hepatotoxicity: histopathological and biochemical analyses in albino rats. Environ Sci Pollut Res Int 2023; 30(8):22034-45. doi: 10.1007/s11356-022-23568-7 [Crossref] [ Google Scholar]
- Olayinka ET, Ore A, Adewole KE, Oyerinde O. Evaluation of the toxicological effects of atrazine-metolachlor in male rats: in vivo and in silico studies. Environ Anal Health Toxicol 2022; 37(3):e2022021-0. doi: 10.5620/eaht.2022021 [Crossref] [ Google Scholar]
- Gao JG, He Y, Hua YL, Chen SX, Jiang Y, Zheng JT, et al. [Atrazine changes meiosis and reduces spermatogenesis in male mice]. Zhonghua Nan Ke Xue 2020;26(11):963-8. [Chinese].
- Nwani CD, Lakra WS, Nagpure NS, Kumar R, Kushwaha B, Srivastava SK. Toxicity of the herbicide atrazine: effects on lipid peroxidation and activities of antioxidant enzymes in the freshwater fish Channa punctatus (Bloch). Int J Environ Res Public Health 2010; 7(8):3298-312. doi: 10.3390/ijerph7083298 [Crossref] [ Google Scholar]
- Hosseinzadeh H, Sadeghnia HR, Ziaee T, Danaee A. Protective effect of aqueous saffron extract (Crocus sativus L) and crocin, its active constituent, on renal ischemia-reperfusion-induced oxidative damage in rats. J Pharm Pharm Sci 2005; 8(3):387-93. [ Google Scholar]
- Mehri S, Abnous K, Khooei A, Mousavi SH, Motamed Shariaty V, Hosseinzadeh H. Crocin reduced acrylamide-induced neurotoxicity in Wistar rat through inhibition of oxidative stress. Iran J Basic Med Sci 2015; 18(9):902-8. [ Google Scholar]
- Altinoz E, Ozmen T, Oner Z, Elbe H, Erdemli ME, Bag HG. Saffron (its active constituent, crocin) supplementation attenuates lipid peroxidation and protects against tissue injury. Bratisl Lek Listy 2016; 117(7):381-7. doi: 10.4149/bll_2016_075 [Crossref] [ Google Scholar]
- Shafahi M, Vaezi G, Shajiee H, Sharafi S, Khaksari M. Crocin inhibits apoptosis and astrogliosis of hippocampus neurons against methamphetamine neurotoxicity via antioxidant and anti-inflammatory mechanisms. Neurochem Res 2018; 43(12):2252-9. doi: 10.1007/s11064-018-2644-2 [Crossref] [ Google Scholar]
- Fani M, Mohammadipour A, Ebrahimzadeh-Bideskan A. The effect of crocin on testicular tissue and sperm parameters of mice offspring from mothers exposed to atrazine during pregnancy and lactation periods: an experimental study. Int J Reprod Biomed 2018; 16(8):519-28. [ Google Scholar]
- Naghizadeh B, Mansouri SM, Vahdati Mashhadian N. Crocin attenuates cisplatin-induced renal oxidative stress in rats. Food Chem Toxicol 2010; 48(10):2650-5. doi: 10.1016/j.fct.2010.06.035 [Crossref] [ Google Scholar]
- Jnaneshwari S, Hemshekhar M, Santhosh MS, Sunitha K, Thushara R, Thirunavukkarasu C. Crocin, a dietary colorant, mitigates cyclophosphamide-induced organ toxicity by modulating antioxidant status and inflammatory cytokines. J Pharm Pharmacol 2013; 65(4):604-14. doi: 10.1111/jphp.12016 [Crossref] [ Google Scholar]
- Timcheh Hariri A, Moallem SA, Mahmoudi M, Hosseinzadeh H. The effect of crocin and safranal, constituents of saffron, against subacute effect of diazinon on hematological and genotoxicity indices in rats. Phytomedicine 2011; 18(6):499-504. doi: 10.1016/j.phymed.2010.10.001 [Crossref] [ Google Scholar]
- van Lieshout M, West CE, van Breemen RB. Isotopic tracer techniques for studying the bioavailability and bioefficacy of dietary carotenoids, particularly beta-carotene, in humans: a review. Am J Clin Nutr 2003; 77(1):12-28. doi: 10.1093/ajcn/77.1.12 [Crossref] [ Google Scholar]
- Zhu L, Dong X, Xie H, Wang J, Wang J, Su J. DNA damage and effects on glutathione-S-transferase activity induced by atrazine exposure in zebrafish (Danio rerio). Environ Toxicol 2011; 26(5):480-8. doi: 10.1002/tox.20575 [Crossref] [ Google Scholar]
- Erdemli ME, Gul M, Altinoz E, Zayman E, Aksungur Z, Bag HG. The protective role of crocin in tartrazine induced nephrotoxicity in Wistar rats. Biomed Pharmacother 2017; 96:930-5. doi: 10.1016/j.biopha.2017.11.150 [Crossref] [ Google Scholar]
- Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 2014; 94(3):909-50. doi: 10.1152/physrev.00026.2013 [Crossref] [ Google Scholar]
- Marchi S, Giorgi C, Suski JM, Agnoletto C, Bononi A, Bonora M. Mitochondria-ros crosstalk in the control of cell death and aging. J Signal Transduct 2012; 2012:329635. doi: 10.1155/2012/329635 [Crossref] [ Google Scholar]
- Andreyev AY, Kushnareva YE, Murphy AN, Starkov AA. Mitochondrial ROS metabolism: 10 years later. Biochemistry (Mosc) 2015; 80(5):517-31. doi: 10.1134/s0006297915050028 [Crossref] [ Google Scholar]
- Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ 2006; 13(9):1423-33. doi: 10.1038/sj.cdd.4401950 [Crossref] [ Google Scholar]
- Wikström M, Gennis RB, Rich PR. Structures of the intermediates in the catalytic cycle of mitochondrial cytochrome c oxidase. Biochim Biophys Acta Bioenerg 2023; 1864(2):148933. doi: 10.1016/j.bbabio.2022.148933 [Crossref] [ Google Scholar]