Int J Drug Res Clin. 2:e2.
doi: 10.34172/ijdrc.2024.e2
Review Article
An Overview of the Application of Green Nature Deep Lung Support, a Natural Product With Herbal Source in Medicine
Atefeh Fakharian 1, 2  , Maryam Sadat Mirenayat 1, 2
, Maryam Sadat Mirenayat 1, 2  , Modjtaba Babazadeh 3, 4
, Modjtaba Babazadeh 3, 4  , Reyhaneh Zahiri 1, 2
, Reyhaneh Zahiri 1, 2  , Seyed Bashir Mirtajani 5, *
, Seyed Bashir Mirtajani 5, *  , Hamidreza Jamaati 2, *
, Hamidreza Jamaati 2, * 
Author information:
1Pulmonary Rehabilitation Research Center (PRRC), National Research Institute of Tuberculosis and Lung Disease (NRITLD), Shahid Beheshti University of Medical Sciences, Tehran, Iran
2Chronic Respiratory Diseases Research Center (CRDRC), National Research Institute of Tuberculosis and Lung Diseases (NRITLD), Shahid Beheshti University of Medical Sciences, Tehran, Iran
3Doctor of Veterinary Medicine, Graduated from Department of Clinical science, Faculty of Urmia, Urmia, Iran
4CEO of GreenNature Company in Iran
5Lung Transplantation Research Center, National Research Institute of Tuberculosis and Lung Diseases (NRITLD), Shahid Beheshti University of Medical Sciences, Tehran, Iran
Abstract
Background:
The use of natural resources, especially medicinal plants, for treating and improving the quality of life has roots as far back as human history. The extensive abilities of plants and the ease of access have led to the use of these huge natural resources in modern medicine. The present study focused on reviewing the component properties of the Green Nature Deep Lung Support as one of the medicinal products with a natural source. Furthermore, while examining some plants’ effects on viral respiratory infections, the scientific bases supporting these claims were discussed as well.
Methods:
Data were collected by searching keywords such as "drug development", "herbal medicine", "infectious disease", "natural product", and "SARS-CoV-2" in Medline, Embase, and Web of Science databases. The main texts of all related articles were reviewed after reviewing the titles of the articles and their abstracts.
Results:
The use of herbal medicines for the treatment and control of viral respiratory infections has been of interest in the past. The effects and benefits of a wide variety of antiviral herbs have been discussed. Green Nature Deep Lung Support product with the extract of plants such as Eriobotrya japonica, Fritillaria cirrhosa, Taraxacum Officinale, Andrographis paniculata, and Echinacea purpurea can be effective in improving respiratory and inflammatory symptoms of various diseases.
Conclusion:
The herbal extracts used in Green Nature Deep Lung Support product have anti-inflammatory and immune-modulating properties and can be influential as a potential treatment in diseases such as COVID-19 that are affected by a cytokine storm.
Keywords: Drug development, Herbal medicine, Infectious disease, Natural product, SARS-COV-2
Copyright and License Information
© 2024 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Please cite this article as follows: Fakharian A, Mirenayat MS, Babazadeh M, Zahiri R, Mirtajani SB, Jamaati H. An overview of the application of green nature deep lung support, a natural product with herbal source in medicine. Int J Drug Res Clin. 2024; 2: e2. doi: 10.34172/ijdrc.2024.e2
Introduction
The outbreak of COVID-19 around the world has been one of the most challenging issues in human society, which has caused the loss of many lives in a short period. During the last 10 years, our world has experienced other similar infectious diseases, whose negative consequences have affected various dimensions of human societies. However, the prevalence and mortality caused by COVID-19 are higher and more severe than these diseases. Vaccination is the most promising approach for controlling COVID-19.1 The performance of many of these vaccines is the transfer of complementary DNA or mitochondrial DNA (mRNA) through liposomes or adenovectors, stimulating the production of spike protein and the neutralization of immunoglobulin G antibodies.2
Although many reports have been published on the positive role of vaccines in reducing the number of patients requiring hospitalization and the burden of COVID-19 infection, this method has also limitations. Investigations have shown that the titer of vaccine antibodies is associated with a significant decrease after the third month.3 On the other hand, vaccinated people are not completely immune, and the possibility of contracting COVID-19 and transmitting the disease to other people still exists. In addition, the mutation created in the spike protein by the virus prevents the correct and targeted functioning of vaccines. Another limitation in the use of vaccines is that vaccines may not be effective in immunocompromised people.4
Therefore, choosing a treatment approach consisting of auxiliary treatments to control the proliferation of the virus and prevent the deterioration of the disease can be considered the most efficient method. The use of nutritional supplements and herbal medicines can prevent damage caused by widespread systemic inflammation caused by COVID-19 by strengthening the immune system.5 It can be mentioned that medicinal herbs and natural products are among the most trusted products in this field. In the past, flavonoid compounds, alkaloids, and polyphenols obtained from plants and mushrooms have been used to strengthen the immune system and using their anti-inflammatory properties.6 Many of these compounds can control the proliferation of the virus and inflammatory responses.6 Some of the bioactive compounds derived from plants have been taken into consideration to control SARS-CoV-2 respiratory infection, including thymoquinone, a-hederin, and nigellidine (obtained from Nigella sativa) 7, quercetin (obtained from Ginko biloba and green tea), and ellagic acid (obtained from Moringa oleifera).8 Further, due to its anti-thrombosis and anti-platelet aggregation effect, Panax ginseng is one of the medicinal plants that has been successfully utilized in the treatment of COVID-19.9 The curcumin, quercetin, luteolin, piperine, and epigallocatechin-3-gallate compounds obtained from Polygonum cuspidatum also played a positive role in the treatment of COVID-19 due to their ability to control the cytokine storm and prevent pulmonary fibrosis.10
Therefore, considering the importance of using these natural resources, the present study aims to review the function of plant extracts used in the Green Nature Deep Lung Support supplement as a natural product that can be effective in improving respiratory diseases.
Methods
The data of this study have been obtained by reviewing English language articles published in the field of herbal natural products in Medline, Embase, and Web of Science databases from 1995 to 2022. Several keywords were searched, including “Drug development”, “Herbal medicine”, “Infectious disease”, “Natural product”, and “SARS-CoV-2”. A total of 253 articles were obtained, of which only 197 articles were accessible in full text. Among them, 127 articles were excluded from the study due to the lack of an understanding of the provided information, and 73 articles were evaluated carefully (Figure 1).
Figure 1.
Diagram of Study Steps
.
Diagram of Study Steps
Results
Andrographis Paniculata
Antibiotics are typically used to treat respiratory infections, but due to resistance to antibiotics, herbal sources can play an effective role in the treatment of these diseases as an auxiliary treatment. A. paniculata is a herbaceous plant of the Acanthaceae family that is found in the tropical and subtropical regions of Asia, Southeast Asia, and India and is known as the king of bitters. Although people of different regions know this plant by different names.11 The extract of this plant, known as andrographolide, has relative solubility in water, and the use of ethanol, chloroform, and ether increases the solubility of this extract. The chemical formula of this substance is C20H30O5. This extract can be obtained by using a 1:1 mixture of dichloromethane and methanol and then by recrystallization, andrographolide is directly separated from the extract.12 Andrographolide has various properties and is found in all parts of the plant, especially the leaves (Figure 2).13 During the last two decades, due to the high variety of the properties of this plant extract, researchers have been attempting to obtain wider biological properties of this plant extract by combining andrographolide with other substances. Investigations have demonstrated that the aqueous or ethanol extract of andrographolide causes a decrease in blood glucose levels in streptozotocin-diabetic and healthy rats.14 Furthermore, this substance can interact with many intracellular components as a bipolar compound and create different biological responses. For example, studies have revealed that A. paniculata polysaccharides with andrographolide improve diabetic nephropathy.14
Figure 2.
Pharmacological Effects of Andrographis paniculata
.
Pharmacological Effects of Andrographis paniculata
According to studies, A. paniculata and its derived compounds have antioxidant properties. The aqueous extract of A. paniculata reduces glutathione by increasing the activity of antioxidant enzymes such as catalase, superoxide dismutase, and glutathione-transferase. This extract prevents lipid peroxidation by reducing thiobarbituric-acid-reactive levels in the liver and kidneys of diabetic rats.15
The expression of the inducible isoforms of nitric oxide (NO) synthase and cyclooxygenase-2 (COX-2) causes excessive production of NO and prostaglandin E2 and drives inflammatory processes of macrophages. The release of pro-inflammatory cytokines by macrophages stimulated with lipopolysaccharide (LPS) induces the inducible isoforms of NO synthase, thus increasing NO production. Investigations have shown that the methanol extract of A. paniculata and andrographolide inhibits NO production by LPS in a concentration-dependent manner.16 Examining the effect of the andrographolide extract on mice demonstrated that this substance can inhibit LPS-induced NO production in RAW 264.7 mouse cell lines. The use of this extract in rats causes the maximum contractile response of the thoracic aorta to phenylephrine to be restored after incubation with LPS, decreasing the average arterial blood pressure. Andrographolide also suppresses interleukin (IL)-2 production and T-cell proliferation in a mixed lymphocytic reaction and inhibits dendritic cell maturation and antigen presentation.17 Several studies have been published on the use of this plant extract, some of which are presented in Table 1.
Table 1.
A Summary of Previous Studies on the Properties of the Andrographis paniculata Extract
|
Type of Activity
|
Authors
|
Article Title
|
Performance
|
| Antioxidant activity |
Lin et al |
Antioxidant, antioedema, and analgesic activities of Andrographis paniculata extracts and their active constituent andrographolide |
Evaluation of the antioxidant activity of Andrographis paniculata water extract showed that this extract increases the activity of antioxidant defense enzymes such as catalase, superoxide dismutase, and glutathione-transferase while decreasing glutathione content. Therefore, this extract has free radical inhibition, xanthine oxidase inhibition, and anti-lipid peroxidation activities. In addition, this extract is anti-edema and anti-pain.18 |
| Diabetes control |
Xu et al |
Synergetic effect of Andrographis paniculata polysaccharide on diabetic nephropathy with andrographolide |
Examination of the andrographolide extract in rats demonstrated that A water-soluble polysaccharide together with andrographolide increases body weight and creatinine clearance rate while decreasing creatinine and urea nitrogen levels in the serum, serum urea, blood glucose, and urinary albumin excretion. This combination can improve the metabolic abnormalities of diabetic rats and prevent or delay the progression of diabetic renal complications, which may be useful as a therapeutic agent to inhibit the progression of diabetes.19 |
| Anti-inflammatory activity |
Chiou et al |
Mechanisms of the suppression of inducible nitric oxide synthase (iNOS) expression in RAW 264.7 cells by andrographolide |
Andrographolide prevented the production of NO caused by LPS and decreased the expression of iNOS in the RAW 264.7 mouse macrophage cell line. Further, the administration of andrographolide to rats restored the maximum contractile response of the thoracic aorta to phenylephrine and decreased the mean arterial blood pressure of rats 20. |
| Anti-cancer activity |
Shi et al |
Inhibition of cell-cycle progression in human colorectal carcinoma Lovo cells by andrographolide |
Andrographolide prevents cell cycle progression in human colorectal cancer Lovo cells by stopping the G1-S phase and inhibits the activity of Cyclin D1/Cdk4 and/or Cyclin A/Cdk2 and Rb phosphorylation by expressing p53, p21 and p16 21. |
| Immunomodulatory activity |
Qin et al |
Andrographolide inhibits the production of TNF-α and IL-2 in lipopolysaccharide-stimulated macrophages: Role of mitogen-activated protein kinases |
Andrographolide decreases TNF-α, IL-12a, and IL-12b mRNA levels. Moreover, this extract can reduce the production of TNF-α and IL-12p70 proteins. Andrographolide prevents ERK1/2 MAP kinase activity and can prevent LPS-induced TNF-α production by suppressing ERK1/2 signaling.22 |
| Hepatoprotective activity |
Singha et al |
Protective activity of andrographolide and arabinogalactan proteins from Andrographis paniculata Nees. against ethanol-induced toxicity in mice |
Examining the toxicity caused by ethanol in mice and evaluating liver and kidney tissues using andrographolide revealed that treating mice with andrographolide and arabinogalactan proteins can minimize the toxicity caused by ethanol. These compounds have bioactive substances that are responsible for protecting the liver against ethanol toxicity.23 |
| Antimicrobial activity |
Zaidan et al |
In vitro screening of five local medicinal plants for antibacterial activity using the disc diffusion method |
Aqueous extract of andrographolide leaves has antimicrobial activity against Gram-positive Staphylococcus aureus, methicillin-resistant Staphylococcus aureus, and Gram-negative Pseudomonas aeruginosa.24 |
| Antiviral activity |
Tang et al |
Screening of anti-dengue activity in the methanolic extracts of medicinal plants. |
Andrographis paniculata methanolic extract has antiviral activity against DENV1-infected Vero E6 cells.25 |
| Effects on cardiovascular disease |
Awang et al |
Cardiovascular activity of labdane diterpenes from Andrographis paniculata in isolated rat hearts. |
The dichloromethane extract of Andrographis paniculata Nees can significantly reduce coronary perfusion pressure and heart rate and cause vasodilation.26 |
| Inhibition of platelet aggregation |
Lu et al |
Suppression of novel NF-κB signaling by andrographolide with a novel mechanism in human platelets: Regulatory roles of the p38 MAPK-hydroxyl radical-ERK2 cascade |
Andrographolide is a novel NF-κB inhibitor that has potent antiplatelet activity by activating the endothelial cyclic GMP pathway of NO (eNOS). This combination has the potential to treat thromboembolic disorders and can be considered for the treatment of various inflammatory diseases.27 |
Note. TNF-α, Tumor necrosis factor-alpha; IL, Interleukin; MAOK, Mitogen-activated protein kinases; NF-κB, nuclear factor-κB; LPS, lipopolysaccharide.
Echinacea
Echinacea is a plant from the Asteraceae family, which includes 11 species of herbaceous and flowering plants. More than 800 products and herbal medicines containing echinacea (herbal medicines, including homeopathic medicines) are currently available on the market.28 Three commercial varieties of this plant include Echinacea purpurea, Echinacea pallida, and Echinacea Angustifolia, whose antibacterial and antiviral properties have been well-established previously. Additionally, the use of these plants is of special interest due to their anti-inflammatory properties and modulation of the immune system.29 Aqueous, alcoholic, and polysaccharide extracts from all parts of the plant, including roots and aerial parts, have the above properties on the cells.30 Echinacea’s active components, including glycoproteins, high molecular weight polysaccharides, phenolic compounds, caffeic acid derivatives, and alkamides, can induce transcription and modulate immune pathways.31 However, using this plant in a situation where the body is in the inflammatory response phase suppresses the immune function.
Echinacea’s inflammatory response through the secretion of inflammatory mediators such as tumor necrosis factor-alpha (TNF-a) and IL-1 beta (IL-1b) and increased phagocytosis by macrophages, resulting in the release of NO, is activated. NO is a signal for the activation of macrophages against various microbes and tumor cells, but when it is produced in excess, it can be harmful to host tissues as well. Therefore, the production of these inflammatory mediators is regulated by the anti-inflammatory mechanisms of factors such as IL-4 and IL-10.32
Echinacea has also anti-cancer effects. The investigation of the effect of using this plant in patients with advanced cancers with extensive metastases has shown that in a number of patients, after immunotherapy, and low-dose cyclophosphamide and thymostimulin, slight clinical and immunological improvements were observed with the use of E. purpurea. In addition, this compound can increase the activity of lymphokine-activated killer cells in patients with advanced liver cancer.33 However, the effect of echinacea immune modulation cannot be separated from other factors and more and more detailed studies are needed in this regard.
Another application of the E. purpurea extract is to improve the severity and duration of various upper respiratory infections such as bronchitis and viral infections such as human and avian influenza viruses, H1N1-type IV, H3N2-type IV, rhinoviruses, and herpes.33 Investigations represented that inulin-type fructans and other fructans are the main immune system-modulating components found in plant extracts.34 What is important in this context is the preparation quality of echinacea products. Herbal products obtained from echinacea are among the best-selling herbal medicines for the treatment and prevention of respiratory infections.35 The function of these products includes a set of anti-inflammatory, antiviral, and immunomodulatory activities.
Eriobotrya japonica
Eriobotrya japonica is an evergreen plant native to subtropical regions, which originates from southeast China. The medicinal use of this plant goes back thousands of years, and its extract has been used to treat cough, chronic bronchitis, inflammation, diabetes, and cancer in traditional Chinese medicine.36 In recent years, scientific evidence has confirmed the active medicinal compounds in the extract of this plant. The extracts obtained from different organs of E. japonica have different compounds. For example, studies have shown that the leaves and flowers of this plant contain significant amounts of phenols and triterpenes. The fruit of this plant has valuable compounds such as carotenoids, flavonoids, sugars, organic acids, and various vitamins, and the kernel also contains various minerals, types of protein, and tannin.37
Eriobotrya japonica leaves have long been used in traditional Chinese medicine to treat ailments such as cough, chronic bronchitis, and asthma. In addition to leaves, the seeds and fruits of this plant have anti-inflammatory activities.38 Many lung diseases are caused by inflammation caused by polysaccharides. Studies have demonstrated that the E. japonica extract enriched with triterpenes, especially ursolic acid, has anti-inflammatory activity in mice.39 Examining the effect of the seed extract of this plant on mice undergoing chemotherapy with mucositis indicated that the use of this extract significantly controlled mucositis, epithelial damage, and bacterial infection.40 The anti-inflammatory effect of the extract obtained from E. japonica is applied through the reduction of pro-inflammatory mediators such as TNF-α, IL-6, IL-8, IL-1β, iNOS, COX-2, and the increase of anti-inflammatory cytokines such as IL-10, followed by the inhibition of NF-κB activation and mitogen-activated protein kinase signaling pathway.39
Other cytotoxic active compounds isolated from E. japonica extract are:
-
Ursolic and oleanolic acids: Inhibition of Molt 4B human lymphoid cell proliferation41
-
Procyanidin oligomers: Inhibition of human salivary gland tumor cells and human squamous cell carcinoma42
-
Epicatechin, procyanidin C-1, procyanidin B-2, and procyanidin oligomer: Inhibitory effect on HSC-2 cells42
-
δ-oleanolic acid, 3-O-(E)-p-coumaroyl tormentic acid, ursolic acid, and betulinic acid: Inhibitory effect on human HL60 cells and the inhibition of DNA topoisomerase I.43
-
Euscaphic acid: Antitumor effect on mouse tumor caused by the 7,12-DMBA initiator and tissue-type plasminogen promoter.44
Fritillaria
Fritillaria is a plant native to the temperate regions of the Northern Hemisphere, which is often detected in Central Asia, the Mediterranean region, and North America. In China, twelve species of this plant are used as natural medicines for respiratory diseases.45 Alkaloids in different species of Fritillaria have different properties such as antitussive, antiasthmatic, chronic obstructive pulmonary disease (COPD) control, and expectorant effects. In addition, the extract of this plant has potential anti-inflammatory, anti-hypertensive, anti-tumor, and bacteriostasis effects.46
Studies have shown that the substances obtained from this plant can be effective for treating coughs without phlegm or chronic coughs.47 According to Fritillaria properties, including anti-cough, anti-pneumonia, expectorant, anti-inflammatory, antioxidant, and anti-asthma effects, it can be considered a suitable option for treating respiratory diseases and improving disorders such as cough, phlegm, bronchial inflammation, adult respiratory distress syndrome, and COPD. Furthermore, it seems that alkaloids present in some species of this plant, considering the effect on the biological processes effective in the inflammatory response, can be considered a potential treatment for respiratory infections such as the emerging COVID-19 infection 48,49 (Figure 3).
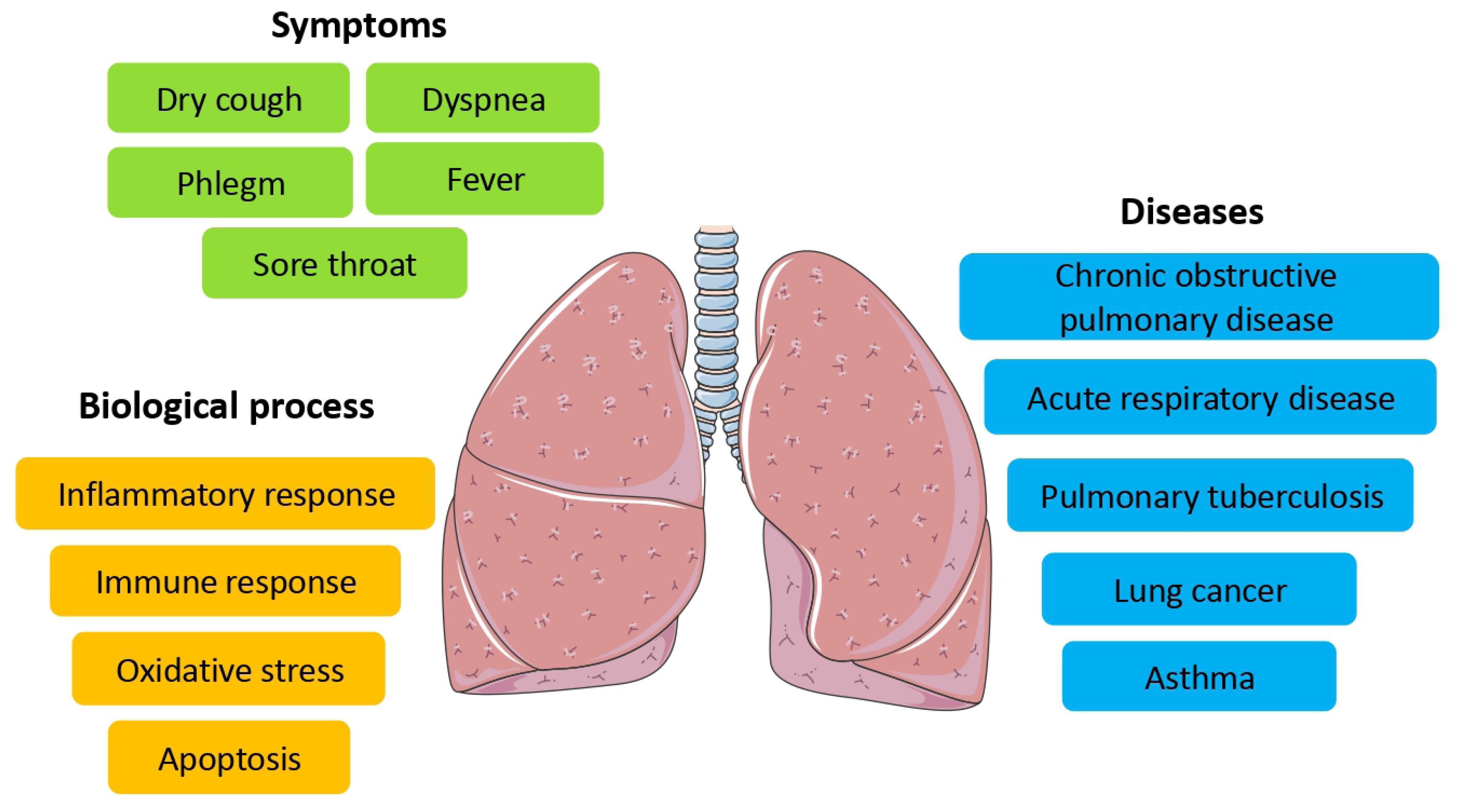
Figure 3.
Summary of Pulmonary Diseases, Symptoms, and Biological Pathways Affected by Fritillaria.48
.
Summary of Pulmonary Diseases, Symptoms, and Biological Pathways Affected by Fritillaria.48
Several species of Fritillaria have anticancer activities in cases such as oral, ovarian, and endometrial keratinocytes, glioblastoma, human promyelocytic leukemia cells, and prostate cancer. One of the active compounds found in many species of Fritillaria is Peimine, which has the effect of reversing drug resistance in tumor cells. Investigating the mechanism of this steroidal alkaloid has represented that the expression of P-glycoprotein in drug-resistant cells is inhibited by increasing the concentration of Peimine.45
Different species of Fritillaria also have anti-inflammatory activities. A study on the ethanol extract of two species, Fritillaria cirrhosa and F. pallidiflora, revealed that consumption of this extract prevents ear edema in mice. Based on previous studies, F. cirrhosa alkaloids also prevented acetic acid-induced capillary permeability, and carrageenan-induced edema and suppressed inflammatory cell recruitment and cytokine production in the bronchoalveolar lavage fluid of mice. Fritillaria bulbs play their anti-inflammatory role by influencing signaling pathways. These pathways include the inhibition of NF-κB, downregulation of inflammatory cytokines, and inhibition of mitogen-activated protein kinases50 (Figure 4). Extracts obtained from some species of Fritillaria have antihypertensive properties. For example, the aqueous extract of F. ussuriensis prevents blood pressure caused by NG-nitro-l-arginine methyl ester by increasing vascular NO production and improving kidney function.46
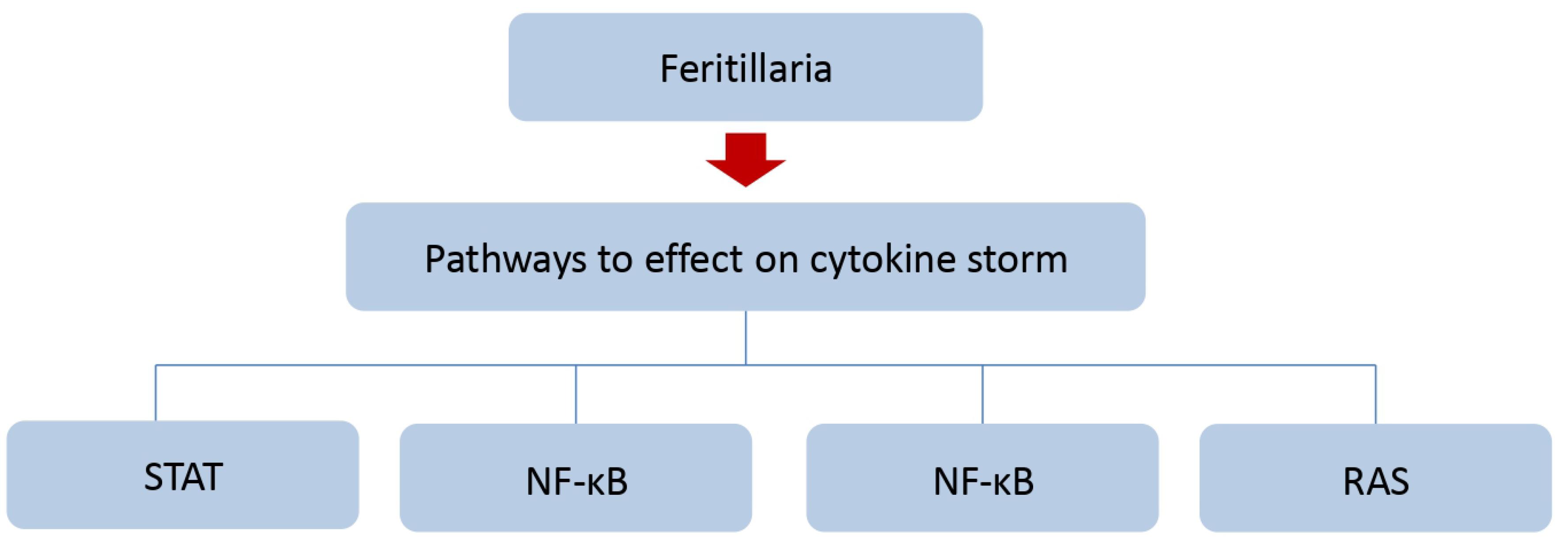
Figure 4.
Fritillaria Pathway for the Treatment of Respiratory Diseases. Note. STAT: Signal transducer and activator of transcription (STAT) protein, NF-κB: Nuclear factor kappa-light-chain-enhancer of activated B cells, MAPK: Mitogen-activated protein kinases, RAS: Renin–angiotensin system
.
Fritillaria Pathway for the Treatment of Respiratory Diseases. Note. STAT: Signal transducer and activator of transcription (STAT) protein, NF-κB: Nuclear factor kappa-light-chain-enhancer of activated B cells, MAPK: Mitogen-activated protein kinases, RAS: Renin–angiotensin system
Another interesting function of Fritillaria is the anticholinesterase activity in some species. Additionally, water and ethanol extracts of this plant have antibiotic activities. For example, F. thunbergii has an inhibitory effect against six strains of Helicobacter pylori.45 In addition to antibiotic activities, some species have antiviral properties. Antiviral effects against the H1N1 influenza virus have been observed in F. thunbergii species.51
Scutellaria baicalensis
Scutellaria baicalensis is a flowering plant from the Lamiaceae family that is native to East Asia and can be found in many European countries. The decoction of the root of this plant, known as Huang-Qin in China, has long been used by the people of this region as a traditional medicine to treat colds and pneumonia.51 Studies have shown that this plant can be effective in treating hepatitis B and C.53 Fufang is another compound derived from this plant that can increase the life span of patients with bronchial squamous cell cancer and non-small-cell lung cancer.54 The S. baicalensis extract can also be effective in the treatment of brain tumors,55 prostate cancer, and head and neck squamous cell carcinoma 55. The action of the aqueous root extract of this plant is through changing the expression of Bcl genes and increasing the inhibitory activity of cyclin-dependent p27 kinase, reducing the expression of the c-myc oncogene and thus inducing apoptosis.57 Some bioactive compounds found in the Scutellaria root extract that have anti-cancer activity are as follows58:
-
Baicalein: Preventing the growth of lymphoma and myeloma cells
-
Wogonoside: Having anticancer effects on acute myeloid leukemia cell lines, increased transcription of phospholipid scramblase 1, and cell cycle regulator and differentiation-related genes
Scutellaria baicalensis has antibacterial and antiviral properties. So far, the antibacterial effect of this plant has been proven on Escherichia coli, Salmonella anatum, Bacillus cereus, Listeria monocytogenes, and Staphylococcus aureus.59 Baicalin can play an anti-HIV-1 role so that only 2 μg/mL of this substance can inhibit up to 90% of reverse transcriptase.60
Today, researchers are attempting to increase the biosynthesis of bioactive flavones such as baicalein, baicalin, wogonin, and wogonoside in S. baicalensis with the help of new technologies. Next-generation sequencing technologies have identified structural genes such as 6-hydroxylase, 8-O-methyltransferase, and 7-O-glucuronosyltransferases responsible for the biosynthesis of flavones.61
Taraxacum officinale
Taraxacum officinale, commonly known as dandelion, is a herbaceous plant of the Asteraceae family that is found in different regions such as Asia, Europe, and North America. In the past, the leaves and young parts of this plant were used as food due to the presence of valuable substances such as fiber, minerals, vitamins, and essential fatty acids.62 In addition, the chemical substances in the flowers, leaves, stems, and roots of this plant, including carotenoids, flavonoids, phenolic acids, sesquiterpene lactones, polysaccharides, triterpenes, and sterols have led to the medicinal use of this plant.63 Taraxacum has different species, among which T. officinale has been studied more due to its various medicinal uses.
Taraxacum officinale has been utilized since the 15th century due to its diuretic properties.64 The extract of this plant has also played a significant role in the treatment and control of renal diseases.65 After the 16th century, the properties of T. officinale in protecting the liver were confirmed, and it was introduced as one of the safest and most practical herbal medicines.66 Further, T. officinale leaf extract is effective in the treatment of disorders such as non-alcoholic fatty liver, protection against sodium, and liver damage caused by dichromate.67
Taraxacum officinale extract can also help strengthen the immune system through processes such as increasing NO, increasing cytokine production, and activating anti-apoptotic Bax/Bc1-2 signaling.68
The antiviral function of the T. officinale extract is performed by inhibiting the transcription and reverse replication of human immunodeficiency virus type 1.69 In addition, the extract of this plant has antiviral activity against influenza, hepatitis B and C, and DENV2.70
Studies indicate that dandelion extract can be considered a potential treatment option for inflammatory processes and tissue damage caused by SARS-CoV-2 infection. As mentioned, the various properties of this plant are valuable due to its bioactive compounds (Table 2). Research has shown that during the process of a cytokine storm caused by COVID-19, taraxasterol contained in this plant can prevent the activation of the inflammatory cycle and subsequent tissue damage by inhibiting interferon-gamma, IL-6, and IL-1B. Cichoric acid in dandelion also reduces lung damage caused by LPSs. Further, cichoric acid inhibits the production of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β by reducing the number of neutrophils and macrophages.71
Table 2.
Examples of Pharmacological Activity and Bioactive Compounds in Dandelion
|
Activity
|
Sample of Bioactive Compound
|
Process
|
| Anti-inflammation |
Taraxasterol and cichoric acid |
Inhibiting pro-inflammatory mediators such as IFN and IL-6 |
| Anti-cancer |
Chlorogenic acid |
Destroying free radicals, preventing DNA damage, inhibiting the induction of carcinogenesis, and increasing the proliferation of cells, including cytotoxic T lymphocytes, natural killer cells, and macrophages |
| Cardioprotection |
Ferulic acid |
Inhibiting apoptosis |
| Effective in HIV treatment |
Chicoric acid |
Increasing life expectancy and preventing co-morbidities |
| Prevent reproduction of SARS-CoV-2 |
Sinapic acid |
Targeting the viral envelope protein |
Note. HIV: Human immunodeficiency virus; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; IFN: Interferon; IL: Interleukin.
Investigations demonstrated that in viral infections of the respiratory system, the damage of epithelial cells due to viral attack is aggravated with simultaneous bacterial infection and mucosal dysfunction. This process has been confirmed in influenza but not yet in COVID-19. Dandelion ethyl acetate extract can inhibit the growth of Gram-positive and Gram-negative bacteria and can thus prevent simultaneous bacterial infection and deteriorate disease conditions.
Olea europaea
Olea europaea is an evergreen tree of the Oleaceae family that is native to tropical and subtropical regions and has long been used in traditional medicine as a diuretic, lowering blood pressure and improving urinary and bladder infections.72 The most common use of this plant is its oil, which has diverse and amazing properties. In traditional medicine, the combination of O. europaea oil with lemon juice is utilized to treat gallstones.73 The oral consumption of the leaves of this plant is considered for the treatment of stomach and intestinal diseases and as a mouth cleaner.74 Further, the decoction of its leaves and dried fruit is applied for diarrhea and treatment of respiratory and urinary tract infections.75
Conclusion
Nowadays, many clinical and laboratory studies revealed that, unlike conventional modern medicines, the use of medicinal plants is often non-toxic or with extremely low toxicity due to their natural origin and long-term use as traditional medicines.
However, it cannot be hidden that many problems can also occur for various reasons such as toxicity due to long-term use, possible medicinal and herbal interactions, and incorrect identification of herbal species. With the spread of COVID-19, due to the limited access to resources and treatment processes, the use of herbal medicines received special attention, and the role of pharmacists was highlighted more than before. On the other hand, the knowledge of pharmacists has been one of the important points to provide efficient drug resources according to patients’ needs. Therefore, the use of herbal medicines with the help of herbal doctors was considered in all countries. These herbal medicinal resources have worked effectively in reducing the symptoms and treating many patients.
Considering the many cases mentioned in the main text of the article about some medicinal plants and their effect on the treatment cycles of upper respiratory tract diseases, it can be indicated that using a suitable formula to combine these plants can have a more positive effect. Our studies demonstrate that some products in the drug market, including Deep Lung Support, have this feature. Therefore, it is suggested that while developing a study method to investigate the effect of such drugs, a study in the form of a clinical trial should be proposed as well. What is clear is the profound and amazing impact of the potential that lies within nature.
Ethics statement
Not applicable.
Disclosure of funding source
None.
Conflict of interests declaration
None to declare.
Acknowledgments
The authors are grateful to all those who contributed to the preparation of this study.
Author contributions
Conceptualization: Maryam Sadat Mirenayat, Atefeh Fakharian.
Data curation: Modjtaba Babazadeh.
Investigation: Modjtaba Babazadeh, Reyhaneh Zahiri.
Methodology: Seyed Bashir Mirtajani.
Supervision: Hamidreza Jamaati, Seyed Bashir Mirtajani.
Writing–original draft: Reyhaneh Zahiri, Maryam Sadat Mirenayat.
Writing–review & editing: Hamidreza Jamaati, Atefeh Fakharian.
References
- Lurie N, Saville M, Hatchett R, Halton J. Developing COVID-19 vaccines at pandemic speed. N Engl J Med 2020; 382(21):1969-73. doi: 10.1056/NEJMp2005630 [Crossref] [ Google Scholar]
- Rijkers GT, Weterings N, Obregon-Henao A, Lepolder M, Dutt T, van Overveld FJ. Antigen presentation of mRNA-based and virus-vectored SARS-CoV-2 vaccines. Vaccines (Basel) 2021; 9(8):848. doi: 10.3390/vaccines9080848 [Crossref] [ Google Scholar]
- Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S. Waning immune humoral response to BNT162b2 COVID-19 vaccine over 6 months. N Engl J Med 2021; 385(24):e84. doi: 10.1056/NEJMoa2114583 [Crossref] [ Google Scholar]
- Galmiche S, Luong Nguyen LB, Tartour E, de Lamballerie X, Wittkop L, Loubet P. Immunological and clinical efficacy of COVID-19 vaccines in immunocompromised populations: a systematic review. Clin Microbiol Infect 2022; 28(2):163-77. doi: 10.1016/j.cmi.2021.09.036 [Crossref] [ Google Scholar]
- Yu R, Zhang S, Zhao D, Yuan Z. A systematic review of outcomes in COVID-19 patients treated with western medicine in combination with traditional Chinese medicine versus western medicine alone. Expert Rev Mol Med 2022; 24:e5. doi: 10.1017/erm.2021.35 [Crossref] [ Google Scholar]
- Brendler T, Al-Harrasi A, Bauer R, Gafner S, Hardy ML, Heinrich M. Botanical drugs and supplements affecting the immune response in the time of COVID-19: implications for research and clinical practice. Phytother Res 2021; 35(6):3013-31. doi: 10.1002/ptr.7008 [Crossref] [ Google Scholar]
- Xu H, Liu B, Xiao Z, Zhou M, Ge L, Jia F. Computational and experimental studies reveal that thymoquinone blocks the entry of coronaviruses into in vitro cells. Infect Dis Ther 2021; 10(1):483-94. doi: 10.1007/s40121-021-00400-2 [Crossref] [ Google Scholar]
- Muhammad S, Hassan SH, Al-Sehemi AG, Shakir HA, Khan M, Irfan M. Exploring the new potential antiviral constituents of Moringa oliefera for SARS-COV-2 pathogenesis: an in silico molecular docking and dynamic studies. Chem Phys Lett 2021; 767:138379. doi: 10.1016/j.cplett.2021.138379 [Crossref] [ Google Scholar]
- Lee YY, Quah Y, Shin JH, Kwon HW, Lee DH, Han JE. COVID-19 and Panax ginseng: targeting platelet aggregation, thrombosis and the coagulation pathway. J Ginseng Res 2022; 46(2):175-82. doi: 10.1016/j.jgr.2022.01.002 [Crossref] [ Google Scholar]
- Peter AE, Sandeep BV, Rao BG, Kalpana VL. Calming the storm: natural immunosuppressants as adjuvants to target the cytokine storm in COVID-19. Front Pharmacol 2020; 11:583777. doi: 10.3389/fphar.2020.583777 [Crossref] [ Google Scholar]
- Kumar RA, Sridevi K, Kumar NV, Nanduri S, Rajagopal S. Anticancer and immunostimulatory compounds from Andrographis paniculata. J Ethnopharmacol 2004; 92(2-3):291-5. doi: 10.1016/j.jep.2004.03.004 [Crossref] [ Google Scholar]
- Rajani M, Shrivastava N, Ravishankara MN. A rapid method for isolation of andrographolide from Andrographis paniculata Nees (Kalmegh). Pharm Biol 2000; 38(3):204-9. doi: 10.1076/1388-0209(200007)3831-sft204 [Crossref] [ Google Scholar]
- Jarukamjorn K, Nemoto N. Pharmacological aspects of Andrographis paniculata on health and its major diterpenoid constituent andrographolide. J Health Sci 2008; 54(4):370-81. doi: 10.1248/jhs.54.370 [Crossref] [ Google Scholar]
- Xu J, Li Z, Cao M, Zhang H, Sun J, Zhao J. Synergetic effect of Andrographis paniculata polysaccharide on diabetic nephropathy with andrographolide. Int J Biol Macromol 2012; 51(5):738-42. doi: 10.1016/j.ijbiomac.2012.06.035 [Crossref] [ Google Scholar]
- Zhang XF, Tan BK. Anti-diabetic property of ethanolic extract of Andrographis paniculata in streptozotocin-diabetic rats. Acta Pharmacol Sin 2000; 21(12):1157-64. [ Google Scholar]
- Batkhuu J, Hattori K, Takano F, Fushiya S, Oshiman K, Fujimiya Y. Suppression of NO production in activated macrophages in vitro and ex vivo by neoandrographolide isolated from Andrographis paniculata. Biol Pharm Bull 2002; 25(9):1169-74. doi: 10.1248/bpb.25.1169 [Crossref] [ Google Scholar]
- Iruretagoyena MI, Sepúlveda SE, Lezana JP, Hermoso M, Bronfman M, Gutiérrez MA. Inhibition of nuclear factor-kappa B enhances the capacity of immature dendritic cells to induce antigen-specific tolerance in experimental autoimmune encephalomyelitis. J Pharmacol Exp Ther 2006; 318(1):59-67. doi: 10.1124/jpet.106.103259 [Crossref] [ Google Scholar]
- Lin FL, Wu SJ, Lee SC, Ng LT. Antioxidant, antioedema and analgesic activities of Andrographis paniculataextracts and their active constituent andrographolide. Phytother Res 2009; 23(7):958-64. doi: 10.1002/ptr.2701 [Crossref] [ Google Scholar]
- Xu J, Li Z, Cao M, Zhang H, Sun J, Zhao J. Synergetic effect of Andrographis paniculata polysaccharide on diabetic nephropathy with andrographolide. Int J Biol Macromol 2012; 51(5):738-42. doi: 10.1016/j.ijbiomac.2012.06.035 [Crossref] [ Google Scholar]
- Chiou WF, Chen CF, Lin JJ. Mechanisms of suppression of inducible nitric oxide synthase (iNOS) expression in RAW 2647 cells by andrographolide. Br J Pharmacol 2000; 129(8):1553-60. doi: 10.1038/sj.bjp.0703191 [Crossref] [ Google Scholar]
- Shi MD, Lin HH, Lee YC, Chao JK, Lin RA, Chen JH. Inhibition of cell-cycle progression in human colorectal carcinoma Lovo cells by andrographolide. Chem Biol Interact 2008; 174(3):201-10. doi: 10.1016/j.cbi.2008.06.006 [Crossref] [ Google Scholar]
- Qin LH, Kong L, Shi GJ, Wang ZT, Ge BX. Andrographolide inhibits the production of TNF-alpha and interleukin-12 in lipopolysaccharide-stimulated macrophages: role of mitogen-activated protein kinases. Biol Pharm Bull 2006; 29(2):220-4. doi: 10.1248/bpb.29.220 [Crossref] [ Google Scholar]
- Singha PK, Roy S, Dey S. Protective activity of andrographolide and arabinogalactan proteins from Andrographis paniculata Nees against ethanol-induced toxicity in mice. J Ethnopharmacol 2007; 111(1):13-21. doi: 10.1016/j.jep.2006.10.026 [Crossref] [ Google Scholar]
- Zaidan MR, Noor Rain A, Badrul AR, Adlin A, Norazah A, Zakiah I. In vitro screening of five local medicinal plants for antibacterial activity using disc diffusion method. Trop Biomed 2005; 22(2):165-70. [ Google Scholar]
- Tang LI, Ling AP, Koh RY, Chye SM, Voon KG. Screening of anti-dengue activity in methanolic extracts of medicinal plants. BMC Complement Altern Med 2012; 12:3. doi: 10.1186/1472-6882-12-3 [Crossref] [ Google Scholar]
- Awang K, Abdullah NH, Hadi AH, Fong YS. Cardiovascular activity of labdane diterpenes from Andrographis paniculata in isolated rat hearts. J Biomed Biotechnol 2012; 2012:876458. doi: 10.1155/2012/876458 [Crossref] [ Google Scholar]
- Lu WJ, Lin KH, Hsu MJ, Chou DS, Hsiao G, Sheu JR. Suppression of NF-κB signaling by andrographolide with a novel mechanism in human platelets: regulatory roles of the p38 MAPK-hydroxyl radical-ERK2 cascade. Biochem Pharmacol 2012; 84(7):914-24. doi: 10.1016/j.bcp.2012.06.030 [Crossref] [ Google Scholar]
- Bauer R. Echinacea: biological effects and active principles. In: Phytomedicines of Europe. American Chemical Society; 1998. p. 140-57. 10.1021/bk-1998-0691.ch012.
- Barrett B. Medicinal properties of Echinacea: a critical review. Phytomedicine 2003; 10(1):66-86. doi: 10.1078/094471103321648692 [Crossref] [ Google Scholar]
- Goldrosen MH, Straus SE. Complementary and alternative medicine: assessing the evidence for immunological benefits. Nat Rev Immunol 2004; 4(11):912-21. doi: 10.1038/nri1486 [Crossref] [ Google Scholar]
- Gertsch J, Schoop R, Kuenzle U, Suter A. Echinacea alkylamides modulate TNF-alpha gene expression via cannabinoid receptor CB2 and multiple signal transduction pathways. FEBS Lett 2004; 577(3):563-9. doi: 10.1016/j.febslet.2004.10.064 [Crossref] [ Google Scholar]
- Park JE, Barbul A. Understanding the role of immune regulation in wound healing. Am J Surg 2004; 187(5A):11S-6S. doi: 10.1016/s0002-9610(03)00296-4 [Crossref] [ Google Scholar]
- Pleschka S, Stein M, Schoop R, Hudson JB. Anti-viral properties and mode of action of standardized Echinacea purpurea extract against highly pathogenic avian influenza virus (H5N1, H7N7) and swine-origin H1N1 (S-OIV). Virol J 2009; 6:197. doi: 10.1186/1743-422x-6-197 [Crossref] [ Google Scholar]
- Lee J-B, Fukai T, Hayashi K, Hayashi T. Characterization of fructan from Chikuyo-Sekko-To, a Kampo prescription, and its antiherpetic activity in vitro and in vivo. Carbohydr Polym 2011; 85(2):408-12. doi: 10.1016/j.carbpol.2011.02.044 [Crossref] [ Google Scholar]
- Sharma M, Arnason JT, Burt A, Hudson JB. Echinacea extracts modulate the pattern of chemokine and cytokine secretion in rhinovirus-infected and uninfected epithelial cells. Phytother Res 2006; 20(2):147-52. doi: 10.1002/ptr.1824 [Crossref] [ Google Scholar]
- Liu Y, Zhang W, Xu C, Li X. Biological activities of extracts from loquat (Eriobotrya japonica Lindl): a review. Int J Mol Sci 2016; 17(12):1983. doi: 10.3390/ijms17121983 [Crossref] [ Google Scholar]
- Zhou C, Chen K, Sun C, Chen Q, Zhang W, Li X. Determination of oleanolic acid, ursolic acid and amygdalin in the flower of Eriobotrya japonica Lindl by HPLC. Biomed Chromatogr 2007; 21(7):755-61. doi: 10.1002/bmc.817 [Crossref] [ Google Scholar]
- Lin JY, Tang CY. Strawberry, loquat, mulberry, and bitter melon juices exhibit prophylactic effects on LPS-induced inflammation using murine peritoneal macrophages. Food Chem 2008; 107(4):1587-96. doi: 10.1016/j.foodchem.2007.10.025 [Crossref] [ Google Scholar]
- Huang Y, Li J, Wang R, Wu Q, Li YH, Yu SC. Effect of triterpene acids of Eriobotrya japonica (Thunb) Lindl leaf on inflammatory cytokine and mediator induction from alveolar macrophages of chronic bronchitic rats. Inflamm Res 2007; 56(2):76-82. doi: 10.1007/s00011-006-5185-0 [Crossref] [ Google Scholar]
- Takuma D, Guangchen S, Yokota J, Hamada A, Onogawa M, Yoshioka S. Effect of Eriobotrya japonica seed extract on 5-fluorouracil-induced mucositis in hamsters. Biol Pharm Bull 2008; 31(2):250-4. doi: 10.1248/bpb.31.250 [Crossref] [ Google Scholar]
- Komiya T, Achiwa Y, Katsuzaki H, Imai K, Sakurai S, Urakawa K. Effect of oleanolic and ursolic acids isolated from loquat (Eriobotrya) on the growth of human lymphoid leukemia cells. Food Sci Technol Int (Tokyo) 1998; 4(4):282-4. doi: 10.3136/fsti9596t9798.4.282 [Crossref] [ Google Scholar]
- Ito H, Kobayashi E, Takamatsu Y, Li SH, Hatano T, Sakagami H. Polyphenols from Eriobotrya japonica and their cytotoxicity against human oral tumor cell lines. Chem Pharm Bull (Tokyo) 2000; 48(5):687-93. doi: 10.1248/cpb.48.687 [Crossref] [ Google Scholar]
- Kikuchi T, Akazawa H, Tabata K, Manosroi A, Manosroi J, Suzuki T. 3-O-(E)-p-coumaroyl tormentic acid from Eriobotrya japonica leaves induces caspase-dependent apoptotic cell death in human leukemia cell line. Chem Pharm Bull (Tokyo) 2011; 59(3):378-81. doi: 10.1248/cpb.59.378 [Crossref] [ Google Scholar]
- Banno N, Akihisa T, Tokuda H, Yasukawa K, Taguchi Y, Akazawa H. Anti-inflammatory and antitumor-promoting effects of the triterpene acids from the leaves of Eriobotrya japonica. Biol Pharm Bull 2005; 28(10):1995-9. doi: 10.1248/bpb.28.1995 [Crossref] [ Google Scholar]
- Wang Y, Hou H, Ren Q, Hu H, Yang T, Li X. Natural drug sources for respiratory diseases from Fritillaria: chemical and biological analyses. Chin Med 2021; 16(1):40. doi: 10.1186/s13020-021-00450-1 [Crossref] [ Google Scholar]
- Wang D, Du Q, Li H, Wang S. The isosteroid alkaloid imperialine from bulbs of Fritillaria cirrhosa mitigates pulmonary functional and structural impairment and suppresses inflammatory response in a COPD-like rat model. Mediators Inflamm 2016; 2016:4192483. doi: 10.1155/2016/4192483 [Crossref] [ Google Scholar]
- Quan Y, Li L, Yin Z, Chen S, Yi J, Lang J. Bulbus fritillariae cirrhosae as a respiratory medicine: is there a potential drug in the treatment of COVID-19?. Front Pharmacol 2021; 12:784335. doi: 10.3389/fphar.2021.784335 [Crossref] [ Google Scholar]
- Cho IH, Lee MJ, Kim JH, Han NY, Shin KW, Sohn Y. Fritillaria ussuriensis extract inhibits the production of inflammatory cytokine and MAPKs in mast cells. Biosci Biotechnol Biochem 2011; 75(8):1440-5. doi: 10.1271/bbb.110076 [Crossref] [ Google Scholar]
- Sharifzadeh K, Farzanegan B, Mirtajani SB, Peyravian F, Jahangirifard A. The potential role of bromelain in the treatment of SARS-COV-2. J Cell Mol Anesth 2020; 5(4):284-5. doi: 10.22037/jcma.v5i4.32113 [Crossref] [ Google Scholar]
- Kim M, Nguyen DV, Heo Y, Park KH, Paik HD, Kim YB. Antiviral activity of Fritillaria thunbergii extract against human influenza virus H1N1 (PR8) in vitro, in ovo and in vivo. J Microbiol Biotechnol 2020; 30(2):172-7. doi: 10.4014/jmb.1908.08001 [Crossref] [ Google Scholar]
- Huang ZH, Xu ZQ. Single Huang-Qin for treatment of bacterial pneumonia. Shizhen Tradit Med Res 1992; 3:106-7. [ Google Scholar]
- Gibo Y. Clinical study of Sho-saiko-to therapy to the Japanese patients with chronic hepatitis type C (CH-C). Prog Med 1994; 14:2027-30. [ Google Scholar]
- Scheck AC, Perry K, Hank NC, Clark WD. Anticancer activity of extracts derived from the mature roots of Scutellariabaicalensis on human malignant brain tumor cells. BMC Complement Altern Med 2006; 6:27. doi: 10.1186/1472-6882-6-27 [Crossref] [ Google Scholar]
- Zhang DY, Wu J, Ye F, Xue L, Jiang S, Yi J. Inhibition of cancer cell proliferation and prostaglandin E2 synthesis by Scutellariabaicalensis. Cancer Res 2003; 63(14):4037-43. [ Google Scholar]
- Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents wogonin, baicalein and baicalin. Cancer Treat Rev 2009; 35(1):57-68. doi: 10.1016/j.ctrv.2008.09.005 [Crossref] [ Google Scholar]
- Chou CC, Pan SL, Teng CM, Guh JH. Pharmacological evaluation of several major ingredients of Chinese herbal medicines in human hepatoma Hep3B cells. Eur J Pharm Sci 2003; 19(5):403-12. doi: 10.1016/s0928-0987(03)00144-1 [Crossref] [ Google Scholar]
- Kumagai T, Müller CI, Desmond JC, Imai Y, Heber D, Koeffler HP. Scutellariabaicalensis, a herbal medicine: anti-proliferative and apoptotic activity against acute lymphocytic leukemia, lymphoma and myeloma cell lines. Leuk Res 2007; 31(4):523-30. doi: 10.1016/j.leukres.2006.08.019 [Crossref] [ Google Scholar]
- Shan B, Cai YZ, Brooks JD, Corke H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int J Food Microbiol 2007; 117(1):112-9. doi: 10.1016/j.ijfoodmicro.2007.03.003 [Crossref] [ Google Scholar]
- Ono K, Nakane H, Fukushima M, Chermann JC, Barré-Sinoussi F. Inhibition of reverse transcriptase activity by a flavonoid compound, 5,6,7-trihydroxyflavone. Biochem Biophys Res Commun 1989; 160(3):982-7. doi: 10.1016/s0006-291x(89)80097-x [Crossref] [ Google Scholar]
- Liu J, Hou J, Jiang C, Li G, Lu H, Meng F. Deep sequencing of the Scutellariabaicalensis Georgi transcriptome reveals flavonoid biosynthetic profiling and organ-specific gene expression. PLoS One 2015; 10(8):e0136397. doi: 10.1371/journal.pone.0136397 [Crossref] [ Google Scholar]
- Escudero NL, De Arellano ML, Fernández S, Albarracín G, Mucciarelli S. Taraxacum officinale as a food source. Plant Foods Hum Nutr 2003; 58(3):1-10. doi: 10.1023/B:QUAL.0000040365.90180.b3 [Crossref] [ Google Scholar]
- Mir MA, Sawhney SS, Jassal MM. Qualitative and quantitative analysis of phytochemicals of Taraxacum officinale. Wudpecker J Pharm Pharmocol 2013; 2(1):1-5. [ Google Scholar]
- Rácz-Kotilla E, Rácz G, Solomon A. The action of Taraxacum officinale extracts on the body weight and diuresis of laboratory animals. Planta Med 1974; 26(3):212-7. doi: 10.1055/s-0028-1099379 [Crossref] [ Google Scholar]
- Yousefi Ghale-Salimi M, Eidi M, Ghaemi N, Khavari-Nejad RA. Inhibitory effects of taraxasterol and aqueous extract of Taraxacum officinale on calcium oxalate crystallization: in vitro study. Ren Fail 2018; 40(1):298-305. doi: 10.1080/0886022x.2018.1455595 [Crossref] [ Google Scholar]
- Faria TC, Nascimento CC, De Vasconcelos SD, Stephens PR. Literature review on the biological effects of Taraxacum officinale plant in therapy. Asian J Pharm Res Dev 2019; 7(3):94-9. doi: 10.22270/ajprd.v7i3.502 [Crossref] [ Google Scholar]
- Cai L, Wan D, Yi F, Luan L. Purification, preliminary characterization and hepatoprotective effects of polysaccharides from dandelion root. Molecules 2017; 22(9):1409. doi: 10.3390/molecules22091409 [Crossref] [ Google Scholar]
- Kim HM, Lee EH, Shin TY, Lee KN, Lee JS. Taraxacum officinale restores inhibition of nitric oxide production by cadmium in mouse peritoneal macrophages. Immunopharmacol Immunotoxicol 1998; 20(2):283-97. doi: 10.3109/08923979809038545 [Crossref] [ Google Scholar]
- Han H, He W, Wang W, Gao B. Inhibitory effect of aqueous dandelion extract on HIV-1 replication and reverse transcriptase activity. BMC Complement Altern Med 2011; 11:112. doi: 10.1186/1472-6882-11-112 [Crossref] [ Google Scholar]
- He W, Han H, Wang W, Gao B. Anti-influenza virus effect of aqueous extracts from dandelion. Virol J 2011; 8:538. doi: 10.1186/1743-422x-8-538 [Crossref] [ Google Scholar]
- Wong C, Law SK, Au DC. A short commentary of the dandelion and its application for COVID-19. Biointerface Res Appl Chem 2023; 13(6):1-7. doi: 10.33263/briac136.539 [Crossref] [ Google Scholar]
- Somova LI, Shode FO, Ramnanan P, Nadar A. Antihypertensive, antiatherosclerotic and antioxidant activity of triterpenoids isolated from Olea europaea, subspecies africana leaves. J Ethnopharmacol 2003; 84(2-3):299-305. doi: 10.1016/s0378-8741(02)00332-x [Crossref] [ Google Scholar]
- Khan Y, Panchal S, Vyas N, Butani A, Kumar V. Olea europaea: a phyto-pharmacological review. Pharmacogn Rev 2007; 1(1):114-8. [ Google Scholar]
- Bellakhdar J, Claisse R, Fleurentin J, Younos C. Repertory of standard herbal drugs in the Moroccan pharmacopoea. J Ethnopharmacol 1991; 35(2):123-43. doi: 10.1016/0378-8741(91)90064-k [Crossref] [ Google Scholar]
- Al-Quraishy S, Othman MS, Dkhil MA, Abdel Moneim AE. Olive (Olea europaea) leaf methanolic extract prevents HCl/ethanol-induced gastritis in rats by attenuating inflammation and augmenting antioxidant enzyme activities. Biomed Pharmacother 2017; 91:338-49. doi: 10.1016/j.biopha.2017.04.069 [Crossref] [ Google Scholar]
- Özcan MM, Matthäus B. A review: benefit and bioactive properties of olive (Olea europaea L) leaves. Eur Food Res Technol 2017; 243(1):89-99. doi: 10.1007/s00217-016-2726-9 [Crossref] [ Google Scholar]