Int J Drug Res Clin. 2023;1:e17.
doi: 10.34172/ijdrc.2023.e17
Original Article
IGF1R and HIF-1α Gene Silencing Inhibits Cancer Cell Growth
Shiva Abolhasani 1, 2  , Sepideh Javadi 3, Hadi Hassannia 4, Ghasem Ghalamfarsa 5, Mohammad Hojjat-Farsangi 6, Farhad Jadidi-Niaragh 1, 3, 7, *
, Sepideh Javadi 3, Hadi Hassannia 4, Ghasem Ghalamfarsa 5, Mohammad Hojjat-Farsangi 6, Farhad Jadidi-Niaragh 1, 3, 7, * 
Author information:
1Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran
3Research Center for Integrative Medicine in Aging, Aging Research Institute, Tabriz University of Medical Sciences, Tabriz, Iran
4Immunogenetic Research Center, Faculty of Medicine and Amol Faculty of Paramedical Sciences, Mazandaran University of Medical Sciences, Sari, Iran
5Cellular and Molecular Research Center, Yasuj University of Medical Sciences, Yasuj, Iran
6Bioclinicum, Department of Oncology-Pathology, Karolinska Institute, Stockholm, Sweden
7Department of Immunology, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Background:
According to several studies, hypoxia-inducible factor-1α (HIF-1α) and insulin-like growth factor 1 receptor (IGF1R) promote cancer progression and drug resistance. The overexpression of IGF1R and HIF-1 α is observed in various cancers, including breast and colon cancers. Thus, we tried to inhibit the progression of tumor cells by blocking IGF1R and HIF-1α.
Methods:
This study used Lipofectamine to transfect small interfering RNAs (siRNAs) into tumor cells. We treated murine mammary carcinoma (4T1) and murine colon carcinoma (CT26) cells with anti-IGF1R and anti-HIF-1 siRNAs. The real-time polymerase chain reaction (PCR) assay studied the impact of siRNA transfection on the mRNA levels of affected factors. Moreover, the viability of cancer cells was investigated by the MTT assay.
Results:
The investigations demonstrated that the mRNA levels of IGF1R and HIF-1α strongly reduced in tumor cell lines following siRNA transfection. Moreover, silencing IGF1R and HIF-1 additively downregulated cell viability in cancer cell lines.
Conclusion:
These findings imply that cancer combination therapy based on IGF1R and HIF-1α targeting may be a novel promising approach for the immunotherapy of breast and colorectal cancers; however, more study is required.
Keywords: IGF1R, HIF-1α, siRNA, Cancer therapy
Copyright and License Information
© 2023 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Cancer is one of our most critical healthcare problems, so 19.3 million new cases and about 10 million cancer-mediated deaths were recorded worldwide in 2020.1 Cancer cells have various features, including replicating indefinitely, escaping from the host’s immune system, and resisting cell death.2-5 Conventional cancer therapies include chemotherapy, radiotherapy, and surgery. These treatments focus on killing cells with low specificity, which can cause serious side effects and toxicities in normal tissues.6,7 Therefore, new treatment strategies are needed to eliminate tumors and minimize side effects. Accordingly, several investigators have tried to target tumor-promoting factors in the tumor microenvironment to enhance anti-tumor immune responses. Cancer cells overexpress several factors that help to progress cancer growth and suppress anti-tumor immune responses. Therefore, blocking these cancer-promoting overexpressed factors can be considered an important therapeutic approach. The insulin-like growth factor (IGF) and hypoxia-inducible factor-1 (HIF-1) are two critical tumor-promoting factors that are overexpressed in the tumor microenvironment. The reciprocal impact of these factors on each other has made them attractive candidates for the simultaneous blockade.
The IGF signaling pathway regulates the growth and development of many tissues by inhibiting apoptosis and stimulating cellular proliferation. The IGF family includes insulin and two ligands, IGF1 and IGF2, that affect cellular activities by binding to cell membrane receptors such as the IGF-1 receptor (IGF1R), the IGF2R, and the insulin receptor, all of which are of the tyrosine kinase receptor family.8 IGF participates in the formation and growth of cancer cells, and it has been related to developing resistance to some standard treatments.9 The overexpression of IGF1R and IGF1 in the blood has been found in several cancer types, including colon, melanoma, prostate, pancreatic, and breast cancers. Studies have displayed that IGF pathway modulators effectively treat several cancers.10 Therefore, targeting the IGF axis could represent a promising strategy for developing anti-cancer therapies.
On the other hand, hypoxia is a common hallmark of cancers and is responsible for the mechanisms of resistance to chemotherapy, immunotherapy, and radiotherapy.11-13 HIF-1 transcription factor plays a pivotal role in the modulation of hypoxia-related factors.14 The α and β subunits form a HIF-1 complex, which is a pivotal factor in tumorigenesis process. HIF-1 regulates the expression and function of several tumor-promoting factors, affecting the cancer cell development and metastasis of tumors.15,16 The increased expression of HIF-1α has been found in various cancers such as breast, colorectal, melanoma, and prostate.17 Interestingly, suppressing HIF-1α with small interfering RNAs (siRNAs) markedly improved anti-tumor effects.18
We hypothesized that suppressing IGF1R and HIF-1 could inhibit tumor cell growth and progression. In the following study, IGF1R and HIF-1α were inhibited by siRNA in murine colon carcinoma (CT26) and murine mammary carcinoma (4T1) cells. Moreover, the impact of silencing IGF1R and HIF-1α on cell viability was also studied.
Methods
Materials
Anti-murine siRNA molecules targeting HIF-1α, IGF1R, and irrelevant control siRNA molecules were bought from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and the MTT Assay Kit was purchased from Merck (Germany).
Cell Lines
The 4T1 and CT26 cell lines were purchased from the Pasteur Institute (Tehran, Iran). Both cell types were cultivated in RPMI-1640 medium with 10% fetal bovine serum, 2% L-glutamine, and pen/strep cocktails. These cell lines were maintained in the incubator at 5% CO2, 37°C, and 95% humidity.
Cell Transfection
In this study, 1 × 104 cells were seeded in a 96-well plate and incubated for 24 hours. According to the manufacturer protocol, the siRNA transfection was done using 60 pM siRNA by Lipofectamine RNAiMax (Invitrogen).
Transfection Efficiency
To evaluate the transfection efficiency of nanoparticle (NP)-encapsulated siRNA, NPs were loaded with phycoerythrin (PE) -conjugated siRNAs and incubated with cancer cells as described above. Subsequently, cells were washed twice and scanned by flow cytometry to evaluate the frequency of cancer cells transfected with NPs.
Cytotoxicity Assay
The MTT test was performed to measure the impact of blocking IGF1R and HIF-1α in 4T1 and CT26 tumor cells. In a 96-well plate, 104 cells were seeded and incubated for 24 hours. The cells were then incubated with Naked siRNA, Lipofectamine, IGF1R siRNA, HIF-1α siRNA, Lipofectamine IGF1R siRNA, Lipofectamine HIF-1α siRNA, and Lipofectamine IGF1R/HIF-1α siRNAs for 24 and 48 hours. The cells’ supernatant was then removed and substituted with Roswell Park Memorial Institute medium enclosing 50 µL MTT mixture (5 mg/mL). After four hours, the supernatant was removed, and 150 μL of dimethyl sulfoxide was added and incubated for 30 minutes. Then, the absorbance of each well was analyzed using spectrophotometers (BioTek, USA) at 570 nm.
Gene Expression Analysis
Following the treatment of cells with different therapeutic groups, total RNAs were purified using the Trizol reagent (Bioneer, South Korea), according to the company’s guidelines. Afterward, cDNA synthesis was done using the RNA samples via a cDNA synthesis kit (Qiagen). The real-time reverse transcription-PCR (qRT-PCR) test used a Light-Cycler 480 RT-PCR system (Roche) and SYBR Green Master Mix (Thermo Fisher Scientific). Finally, the relative mRNA expression of IGF1R and HIF-1α was quantified using the 2-∆∆CT formula. Primer sequences are demonstrated in Table 1.19,20
Table 1.
Primer sequences
|
Gene Name
|
Forward
|
Reverse
|
| β-actin |
5′-GGTCATCACTATTGGCAACG-3′ |
5′-ACGGATGTCAACGTCACACT-3′ |
| IGF1R |
5′-GTGGGGGCTCGTGTTTCTC-3′ |
5′-TGATCACCGTGCAGTTTTCCA-3′ |
| HIF-1a |
5′-AGCAGGAATTGGAACATTATTGCAG-3′ |
5′-TGTGGTAATCCACTCTCATCCATTG-3′ |
Statistical Analysis
Statistical analyses were done by GraphPad Prism V8 software and the one-way ANOVA test.
P value < 0.05 was also considered significant.
Results
Nanoparticles Markedly Transfected Cancer Cells
To evaluate the transfection potential of NPs, we incubated cancer cells with NPs loaded with PE-labeled siRNAs and assessed the transfection efficiency using the flow cytometry assay. As shown in Figure 1, about 70% of cells were successfully transfected with NPs, which is an acceptable transfection rate.
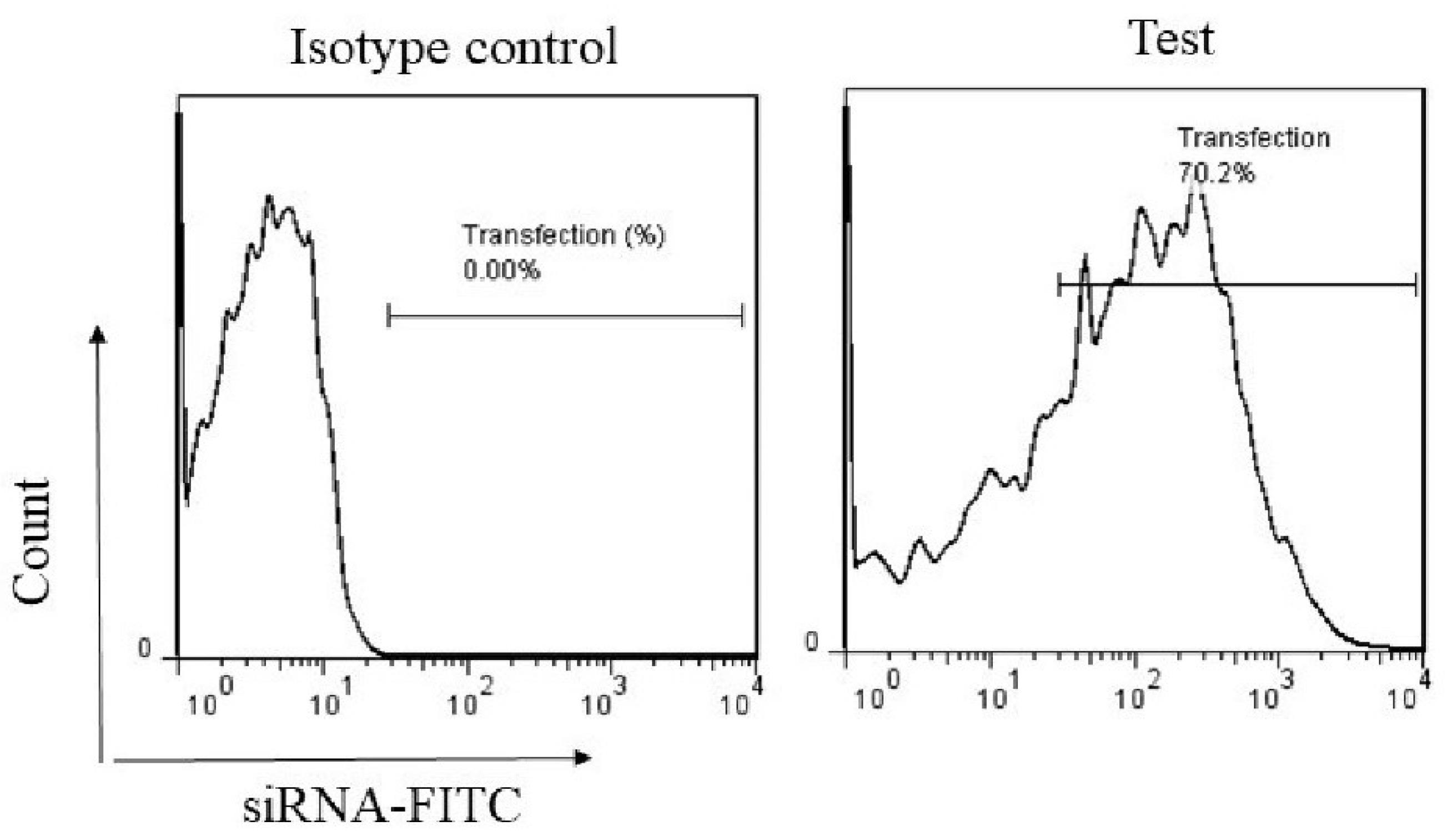
Figure 1.
NPs Encapsulating siRNA Molecules Efficiently Transfected Cancer Cells. Flow cytometry analysis was used to investigate the transfection efficiency of NPs. Cancer cells were treated with PE-labeled siRNAs and incubated for 24 hours. Following cell washing, cancer cells were scanned by flow cytometry to assess the percentage of cells transfected with NPs containing siRNAs. Note. NPs: Nanoparticles; siRNAs: Small interfering RNAs
.
NPs Encapsulating siRNA Molecules Efficiently Transfected Cancer Cells. Flow cytometry analysis was used to investigate the transfection efficiency of NPs. Cancer cells were treated with PE-labeled siRNAs and incubated for 24 hours. Following cell washing, cancer cells were scanned by flow cytometry to assess the percentage of cells transfected with NPs containing siRNAs. Note. NPs: Nanoparticles; siRNAs: Small interfering RNAs
The siRNA Transfection Meaningfully Silenced the Expression of Insulin-like Growth Factor 1 Receptor and Hypoxia-inducible Factor-1α in Cancer Cell Lines
Following the treatment of cells with different therapeutic groups, the mRNA levels of IGF1R and HIF-1α were evaluated using qPCR to investigate the efficiency of gene silencing. Cells treated with Naked siRNA and Lipofectamine alone did not exhibit significant changes in the mRNA levels. Moreover, mRNA levels did not change when IGF1R siRNA and HIF-1α siRNA were used alone, which indicates that cells need a carrier to transfect siRNA. However, treatment of cells with Lipofectamine IGF1R siRNA and Lipofectamine HIF-1α siRNA markedly decreased the mRNA levels of IGF1R and HIF-1α compared to other treatment groups. As depicted in Figure 2, the most significant decrease in mRNA expression levels was detected in cells incubated with Lipofectamine IGF1R/HIF-1α siRNAs. This indicates a reciprocal relationship between the molecules, the blockade of which one affects the expression of another.
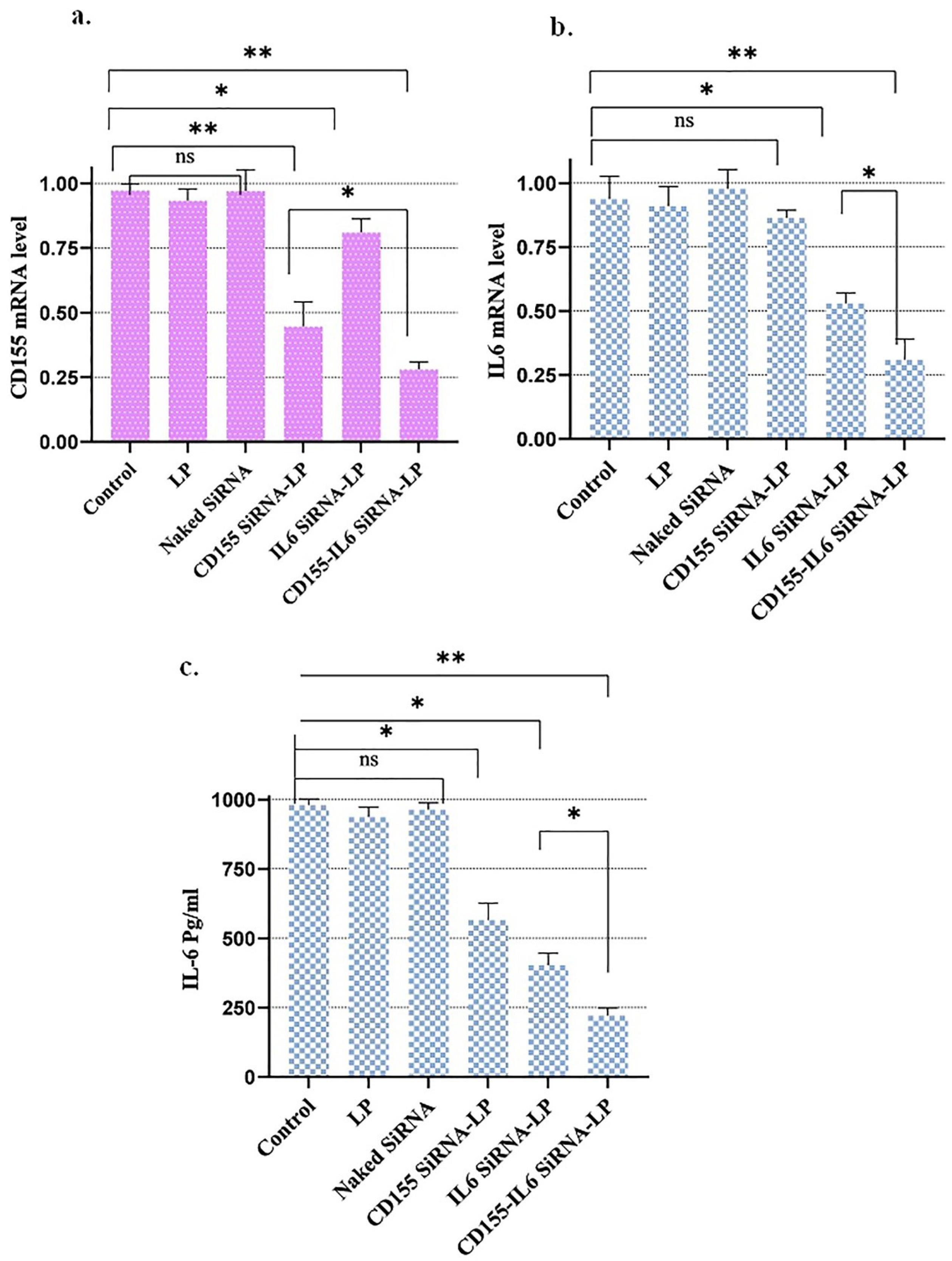
Figure 2.
The mRNA and protein expression levels of CD155 and IL-6. As measured by qPCR assay, the transformation of cancer cell lines by siRNA downregulated HIF-1 and IGF1R mRNA levels. Bar charts demonstrate the mean values of gene expression ± SEM. Note. qPCR: Real-time polymerase chain reaction; HIF-1: Hypoxia-inducible factor-1; IGF1R: Insulin-like growth factor-1 receptor; SEM: Standard error of measurement; * P < 0.05; ** P < 0.01.
.
The mRNA and protein expression levels of CD155 and IL-6. As measured by qPCR assay, the transformation of cancer cell lines by siRNA downregulated HIF-1 and IGF1R mRNA levels. Bar charts demonstrate the mean values of gene expression ± SEM. Note. qPCR: Real-time polymerase chain reaction; HIF-1: Hypoxia-inducible factor-1; IGF1R: Insulin-like growth factor-1 receptor; SEM: Standard error of measurement; * P < 0.05; ** P < 0.01.
Silencing of Insulin-like Growth Factor 1 Receptor and Hypoxia-inducible Factor-1α Expression Induced Apoptosis in Cancer Cell Lines
The MTT assay was used to analyze the cytotoxic impact of various therapeutic groups on the viability of tumor cells after 24 and 48 hours of incubation with the therapeutic groups. The results are presented in Figure 3.
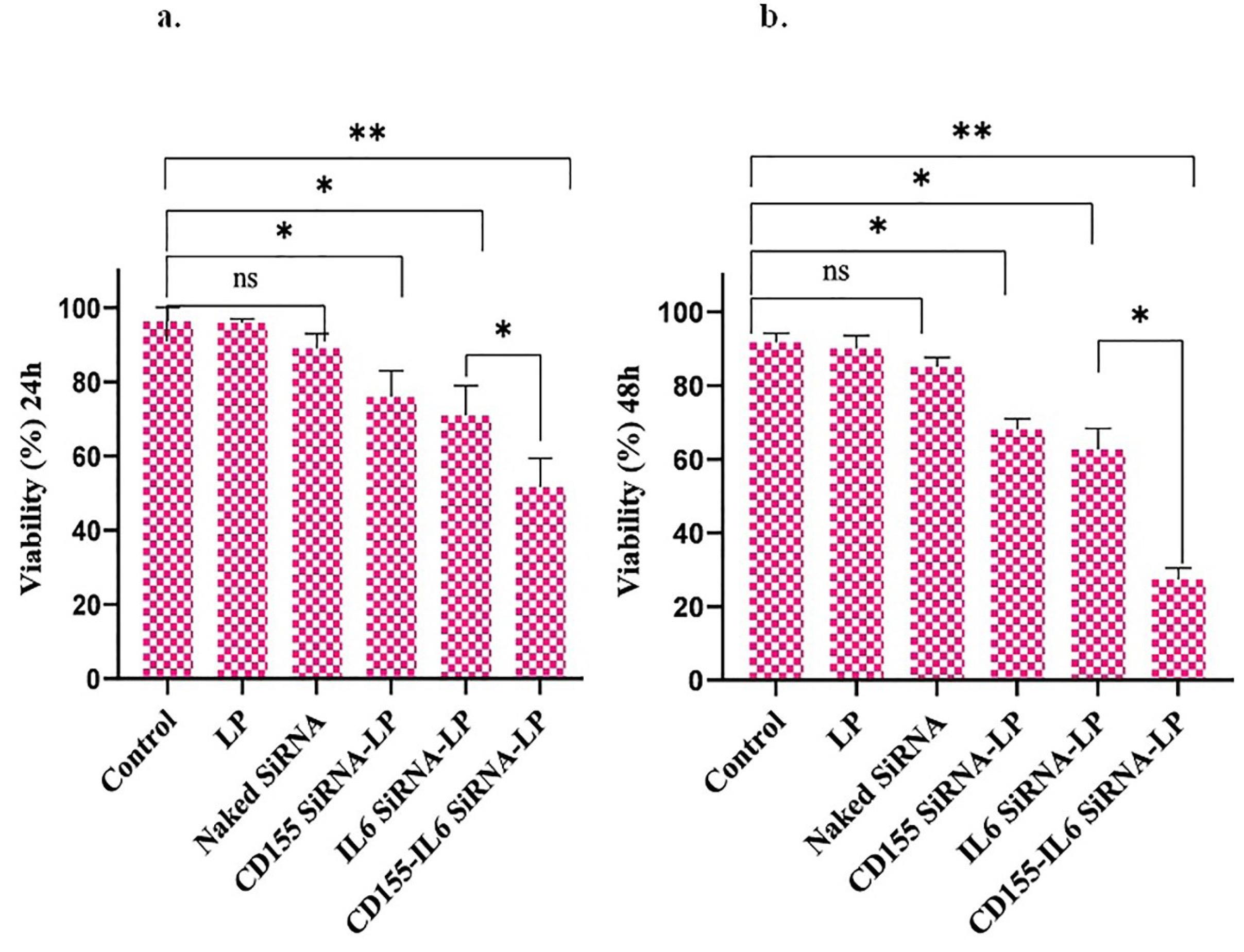
Figure 3.
Viability assay in cancer cells. The downregulation of HIF-1 and IGF1R induces cell death in cancer cells. Bar charts demonstrate the mean values of cell viability ± SEM. Note. HIF-1 α: Hypoxia-inducible factor-1α; IGF1R: Insulin-like growth factor-1 receptor; SEM: Standard error of measurement; * P < 0.05; ** P < 0.01.
.
Viability assay in cancer cells. The downregulation of HIF-1 and IGF1R induces cell death in cancer cells. Bar charts demonstrate the mean values of cell viability ± SEM. Note. HIF-1 α: Hypoxia-inducible factor-1α; IGF1R: Insulin-like growth factor-1 receptor; SEM: Standard error of measurement; * P < 0.05; ** P < 0.01.
Cells treated with Naked siRNA and Lipofectamine alone did not affect the viability of both cell lines, which shows their safety. Furthermore, IGF1R siRNA and HIF-1α siRNA had no effect on viability, indicating the carrier requirement for cellular transfection. On the other hand, Lipofectamine IGF1R siRNA and Lipofectamine HIF-1α siRNA significantly decreased cell viability. In addition, the highest apoptosis rate was found in cells treated with the Lipofectamine IGF1R/HIF-1α siRNAs group compared to the control group, and the apoptotic effects were additive.
Discussion
Identifying the pathways in tumor progression is critical for developing effective therapeutic strategies. IGF is an essential factor in the oncogenesis of most solid cancers. This pathway has a role in cancer cell proliferation and metastasis, which makes it an attractive therapeutic target.21 Hypoxia serves as a selective pressure, leading tumor biology to become aggressive. HIF-1α promotes cancer progression by increasing the expression of factors that modulate glucose metabolism, stem cell maintenance, cell proliferation, cell invasion and metastasis, angiogenesis, and drug resistance.22,23 Therefore, inhibiting HIF-1α may be an effective strategy for cancer treatment.
Evidence shows that IGF-1 synergizes with HIF-1α to promote tumor growth.24,25 IGF1R and HIF-1 may also upregulate vascular endothelial growth factor, provoking angiogenesis.26,27
The current research investigated the effects of inhibiting IGF1R and HIF-1α. Lipofectamine was utilized to transfect IGF1R and HIF-1α siRNA into tumor cells. The qRT-PCR findings showed that siRNAs targeting IGF1R and HIF-1α were effectively transfected to tumor cells as IGF1R and HIF-1α mRNA levels were markedly suppressed. Moreover, treatment with Naked siRNA, IGF1R siRNA, and HIF-1α alone did not affect IGF1R and HIF-1α.
After treating cancer cell lines with different therapeutic groups for 24 or 48 hours, MTT assays were conducted (Figure 2). The results suggested that cells given Lipofectamine IGF1R siRNA and Lipofectamine HIF-1α siRNA alone show significant apoptosis rates. Moreover, cells treated with Lipofectamine IGF1R/HIF-1α siRNAs combination therapy exhibited the highest apoptosis rate. Furthermore, cell viability was time-dependent since 48-hour treatment induced more apoptosis than 24-hour treatment. However, it should be noted that finding the mechanism by which this therapeutic strategy causes cell death is significant, which was not investigated in this study and should be explored in future studies.
Similarly, our findings were consistent with previous studies on the role of IGF1R and HIF-1α inhibition. For example, Durfort et al found that silencing IGF1R using 2′-O-methyl-modified siRNAs blocks the cell cycle and reduces cell proliferation in murine breast cancer cell lines.28 Subramani et al showed that blocking IGF1R inhibits growth and metastasis in pancreatic cancer cell lines.29 Moreover, Hajizadeh et al demonstrated that silencing of HIF-1α /CD73 markedly decreases the expression of CD73 and HIF-1α levels, cell growth, and angiogenesis in cancer cells.20 The inhibition of HIF-1α by cationic mixed micellar nanoparticles containing anti-HIF-1α siRNA also suppressed proliferation, angiogenesis, and migration in PC3 prostate cancer cells. It also makes cancer cells more sensitive to doxorubicin in vitro and in vivo.30
Conclusion
In sum, our results indicate that blocking IGF1R and HIF-1 causes an additive anti-tumor response and inhibits tumor cell growth and progression of breast and colon cancer cells.
Ethics statement
The Ethics Committee of Tabriz University of Medical Sciences has approved this study (Ethics No. IR.TBZMED.VCR.REC.1401.256).
Disclosure of funding source
Financial support for this study was by the Tabriz University of Medical Sciences (grant number: 69991).
Conflict of interests declaration
There is nothing to declare.
Acknowledgments
We would like to thank the Tabriz University of Medical Sciences for providing financial support for this study (Grant number: 69991).
Data availability statement
The data supporting this study’s findings are available on request from the corresponding author following the reasonable request.
Consent for publication
I hereby provide consent for the publication of this article, including any accompanying images or data contained within the manuscript.
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71(3):209-49. doi: 10.3322/caac.21660 [Crossref] [ Google Scholar]
- Bhutia SK, Maiti TK. Targeting tumors with peptides from natural sources. Trends Biotechnol 2008; 26(4):210-7. doi: 10.1016/j.tibtech.2008.01.002 [Crossref] [ Google Scholar]
- Dreesen O, Brivanlou AH. Signaling pathways in cancer and embryonic stem cells. Stem Cell Rev 2007; 3(1):7-17. doi: 10.1007/s12015-007-0004-8 [Crossref] [ Google Scholar]
- Fernald K, Kurokawa M. Evading apoptosis in cancer. Trends Cell Biol 2013; 23(12):620-33. doi: 10.1016/j.tcb.2013.07.006 [Crossref] [ Google Scholar]
- Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol 2006; 6(10):715-27. doi: 10.1038/nri1936 [Crossref] [ Google Scholar]
- Amit D, Hochberg A. Development of targeted therapy for bladder cancer mediated by a double promoter plasmid expressing diphtheria toxin under the control of H19 and IGF2-P4 regulatory sequences. J Transl Med 2010; 8:134. doi: 10.1186/1479-5876-8-134 [Crossref] [ Google Scholar]
- Wu D, Gao Y, Qi Y, Chen L, Ma Y, Li Y. Peptide-based cancer therapy: opportunity and challenge. Cancer Lett 2014; 351(1):13-22. doi: 10.1016/j.canlet.2014.05.002 [Crossref] [ Google Scholar]
- LeRoith D, Roberts CT Jr. The insulin-like growth factor system and cancer. Cancer Lett 2003; 195(2):127-37. doi: 10.1016/s0304-3835(03)00159-9 [Crossref] [ Google Scholar]
- Denduluri SK, Idowu O, Wang Z, Liao Z, Yan Z, Mohammed MK. Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Genes Dis 2015; 2(1):13-25. doi: 10.1016/j.gendis.2014.10.004 [Crossref] [ Google Scholar]
- Jin M, Buck E, Mulvihill MJ. Modulation of insulin-like growth factor-1 receptor and its signaling network for the treatment of cancer: current status and future perspectives. Oncol Rev 2013; 7(1):e3. doi: 10.4081/oncol.2013.e3 [Crossref] [ Google Scholar]
- Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer 2002; 2(1):38-47. doi: 10.1038/nrc704 [Crossref] [ Google Scholar]
- Shannon AM, Bouchier-Hayes DJ, Condron CM, Toomey D. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat Rev 2003; 29(4):297-307. doi: 10.1016/s0305-7372(03)00003-3 [Crossref] [ Google Scholar]
- Daniel SK, Sullivan KM, Labadie KP, Pillarisetty VG. Hypoxia as a barrier to immunotherapy in pancreatic adenocarcinoma. Clin Transl Med 2019; 8(1):10. doi: 10.1186/s40169-019-0226-9 [Crossref] [ Google Scholar]
- Samec M, Liskova A, Koklesova L, Mersakova S, Strnadel J, Kajo K. Flavonoids targeting HIF-1: implications on cancer metabolism. Cancers (Basel) 2021; 13(1):130. doi: 10.3390/cancers13010130 [Crossref] [ Google Scholar]
- Ma Z, Xiang X, Li S, Xie P, Gong Q, Goh BC. Targeting hypoxia-inducible factor-1, for cancer treatment: recent advances in developing small-molecule inhibitors from natural compounds. Semin Cancer Biol 2022; 80:379-90. doi: 10.1016/j.semcancer.2020.09.011 [Crossref] [ Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer 2003; 3(10):721-32. doi: 10.1038/nrc1187 [Crossref] [ Google Scholar]
- Keith B, Johnson RS, Simon MC. HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer 2011; 12(1):9-22. doi: 10.1038/nrc3183 [Crossref] [ Google Scholar]
- Fathi M, Bahmanpour S, Barshidi A, Rasouli H, Karoon Kiani F, Mahmoud Salehi Khesht A. Simultaneous blockade of TIGIT and HIF-1α induces synergistic anti-tumor effect and decreases the growth and development of cancer cells. Int Immunopharmacol 2021; 101(Pt A):108288. doi: 10.1016/j.intimp.2021.108288 [Crossref] [ Google Scholar]
- Lofqvist C, Willett KL, Aspegren O, Smith AC, Aderman CM, Connor KM. Quantification and localization of the IGF/insulin system expression in retinal blood vessels and neurons during oxygen-induced retinopathy in mice. Invest Ophthalmol Vis Sci 2009; 50(4):1831-7. doi: 10.1167/iovs.08-2903 [Crossref] [ Google Scholar]
- Hajizadeh F, Moghadaszadeh Ardebili S, Baghi Moornani M, Masjedi A, Atyabi F, Kiani M. Silencing of HIF-1α/CD73 axis by siRNA-loaded TAT-chitosan-spion nanoparticles robustly blocks cancer cell progression. Eur J Pharmacol 2020; 882:173235. doi: 10.1016/j.ejphar.2020.173235 [Crossref] [ Google Scholar]
- Wang P, Mak VC, Cheung LW. Drugging IGF-1R in cancer: new insights and emerging opportunities. Genes Dis 2023; 10(1):199-211. doi: 10.1016/j.gendis.2022.03.002 [Crossref] [ Google Scholar]
- Yeo EJ, Chun YS, Park JW. New anticancer strategies targeting HIF-1. Biochem Pharmacol 2004; 68(6):1061-9. doi: 10.1016/j.bcp.2004.02.040 [Crossref] [ Google Scholar]
- Semenza GL. HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev 2010; 20(1):51-6. doi: 10.1016/j.gde.2009.10.009 [Crossref] [ Google Scholar]
- Catrina SB, Botusan IR, Rantanen A, Catrina AI, Pyakurel P, Savu O. Hypoxia-inducible factor-1alpha and hypoxia-inducible factor-2alpha are expressed in Kaposi sarcoma and modulated by insulin-like growth factor-I. Clin Cancer Res 2006; 12(15):4506-14. doi: 10.1158/1078-0432.ccr-05-2473 [Crossref] [ Google Scholar]
- Catrina SB, Lewitt M, Massambu C, Dricu A, Grünler J, Axelson M. Insulin-like growth factor-I receptor activity is essential for Kaposi’s sarcoma growth and survival. Br J Cancer 2005; 92(8):1467-74. doi: 10.1038/sj.bjc.6602408 [Crossref] [ Google Scholar]
- Fukuda R, Hirota K, Fan F, Jung YD, Ellis LM, Semenza GL. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J Biol Chem 2002; 277(41):38205-11. doi: 10.1074/jbc.M203781200 [Crossref] [ Google Scholar]
- Treins C, Giorgetti-Peraldi S, Murdaca J, Monthouël-Kartmann MN, Van Obberghen E. Regulation of hypoxia-inducible factor (HIF)-1 activity and expression of HIF hydroxylases in response to insulin-like growth factor I. Mol Endocrinol 2005; 19(5):1304-17. doi: 10.1210/me.2004-0239 [Crossref] [ Google Scholar]
- Durfort T, Tkach M, Meschaninova MI, Rivas MA, Elizalde PV, Venyaminova AG. Small interfering RNA targeted to IGF-IR delays tumor growth and induces proinflammatory cytokines in a mouse breast cancer model. PLoS One 2012; 7(1):e29213. doi: 10.1371/journal.pone.0029213 [Crossref] [ Google Scholar]
- Subramani R, Lopez-Valdez R, Arumugam A, Nandy S, Boopalan T, Lakshmanaswamy R. Targeting insulin-like growth factor 1 receptor inhibits pancreatic cancer growth and metastasis. PLoS One 2014; 9(5):e97016. doi: 10.1371/journal.pone.0097016 [Crossref] [ Google Scholar]
- Liu XQ, Xiong MH, Shu XT, Tang RZ, Wang J. Therapeutic delivery of siRNA silencing HIF-1 alpha with micellar nanoparticles inhibits hypoxic tumor growth. Mol Pharm 2012; 9(10):2863-74. doi: 10.1021/mp300193f [Crossref] [ Google Scholar]