Int J Drug Res Clin. 2023;1:e25.
doi: 10.34172/ijdrc.2023.e25
Review Article
Seizure as a Manifestation of Fingolimod Rebound Syndrome: A Case Report and Literature Review
Samaneh Hosseini 1  , Nasrin Forghani 1
, Nasrin Forghani 1  , Sina Hassannezhad 2
, Sina Hassannezhad 2  , Hanie Karimi 3
, Hanie Karimi 3  , Reza Mosaddeghi Heris 1, *
, Reza Mosaddeghi Heris 1, * 
Author information:
1Neurosciences Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran
3School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
Abstract
Background:
Multiple sclerosis (MS) is a chronic autoimmune disease that affects the central nervous system, leading to inflammation and damage to the protective covering of nerve fibers. MS patients have an increased risk of developing epileptic seizures compared to the general population. The incidence of seizures in MS patients varies across different studies, ranging from 0.5% to 8.3%. Fingolimod is an oral medication used for the treatment of relapsing-remitting MS. It works by inhibiting sphingosine 1-phosphate receptors, which helps to reduce inflammation and prevent immune cells from attacking the nervous system. However, discontinuation of Fingolimod therapy can lead to withdrawal symptoms and potentially increase the risk of epileptic seizures in MS patients.
Methods:
In this case report, we aimed to present a rare manifestation of seizure as a rebound activity following discontinuation of Fingolimod therapy. We reviewed the existing literature to explore the association between Fingolimod and seizures in MS patients. Relevant studies and case reports were identified through a comprehensive search of electronic databases.
Results:
Our case report describes a patient who experienced a seizure after discontinuing Fingolimod therapy. This suggests that abrupt withdrawal of Fingolimod may trigger rebound activity, including seizures, in some MS patients. The literature review revealed several other cases reporting seizures or convulsions associated with Fingolimod use in MS patients. The incidence of seizures in these cases varied, highlighting the need for further research to determine the exact risk.
Conclusion:
Fingolimod discontinuation can lead to rebound activity, including seizures, in some MS patients. It is important for healthcare professionals to be aware of this potential risk and carefully manage the discontinuation process. Further research is needed to better understand the incidence and mechanisms underlying Fingolimod-associated seizures in MS patients.
Keywords: Fingolimod hydrochloride, Multiple sclerosis, Seizure
Copyright and License Information
© 2023 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Multiple sclerosis (MS) is an autoimmune inflammatory illness that affects the central nervous system (CNS) and causes plaque formation and breakdown of the myelin sheath, resulting in axonal destruction. Relapsing-remitting multiple sclerosis (RRMS) is the most frequently occurring type of MS, which is marked by recurrent episodes of neurological impairment.1,2
Fingolimod is a sphingosine 1-phosphate receptor modulator used as a single-agent disease-modifying therapy in RRMS to alleviate the likelihood of disease exacerbation and postpone the onset of physical impairment or disability.3 Fingolimod is suggested to provide protective effects against immune-induced axonal deterioration by inhibiting the emigration of lymphocytes from the thymus and lymph nodes, hence, lowering the abundance of these immune cells in the circulation.4
Despite its benefits for patients with MS, fingolimod is strongly advised to be discontinued in conditions such as pregnancy, the development of severe side effects, and breakthrough in disease activity. When lymphocytes reappear in the circulation and the CNS, 4–12 weeks after medication discontinuation, the disease begins to recur.5 Accordingly, we aimed to report a case who developed seizure as an uncommon manifestation associated with fingolimod discontinuation.
Case Presentation
The patient was a 47-year-old woman diagnosed with RRMS when she was 41 about six years ago. The first clinical manifestation was right-sided optic neuritis, and she was treated with a first-line injectable agent. The patient had been receiving fingolimod (Marela 0.5 mg tablet, Modava Pharmaceuticals, Tehran, Iran) for two years by the time she was included in this report. However, due to the ongoing progression of her disease secondary-progressive multiple sclerosis (SPMS), she was prescribed Rituximab, an anti-CD20 monoclonal antibody.
Within two weeks after the discontinuation of fingolimod, the patient developed a cluster of tonic-colonic seizures. All the episodes were generalized tonic colonic without focal onset. The duration of each attack was between 3 to 5 minutes, and she was responsive between attacks and they were controlled in the Emergency department by Levetiracetam. Initial brain computed tomography (CT) did not reveal any pathology. Subsequent digital EEG showed moderate hypersynchronia with generalized spike and wave epileptiform discharges, and metabolic panel and U/A toxicology were within normal limits. Her expanded disability status scale (EDSS) had increased from 5.5 to 7, and there was now a new region of contrast enhancement on her brain magnetic resonance imaging (MRI), indicating newly developed neuronal injury (Figures 1 and 2).
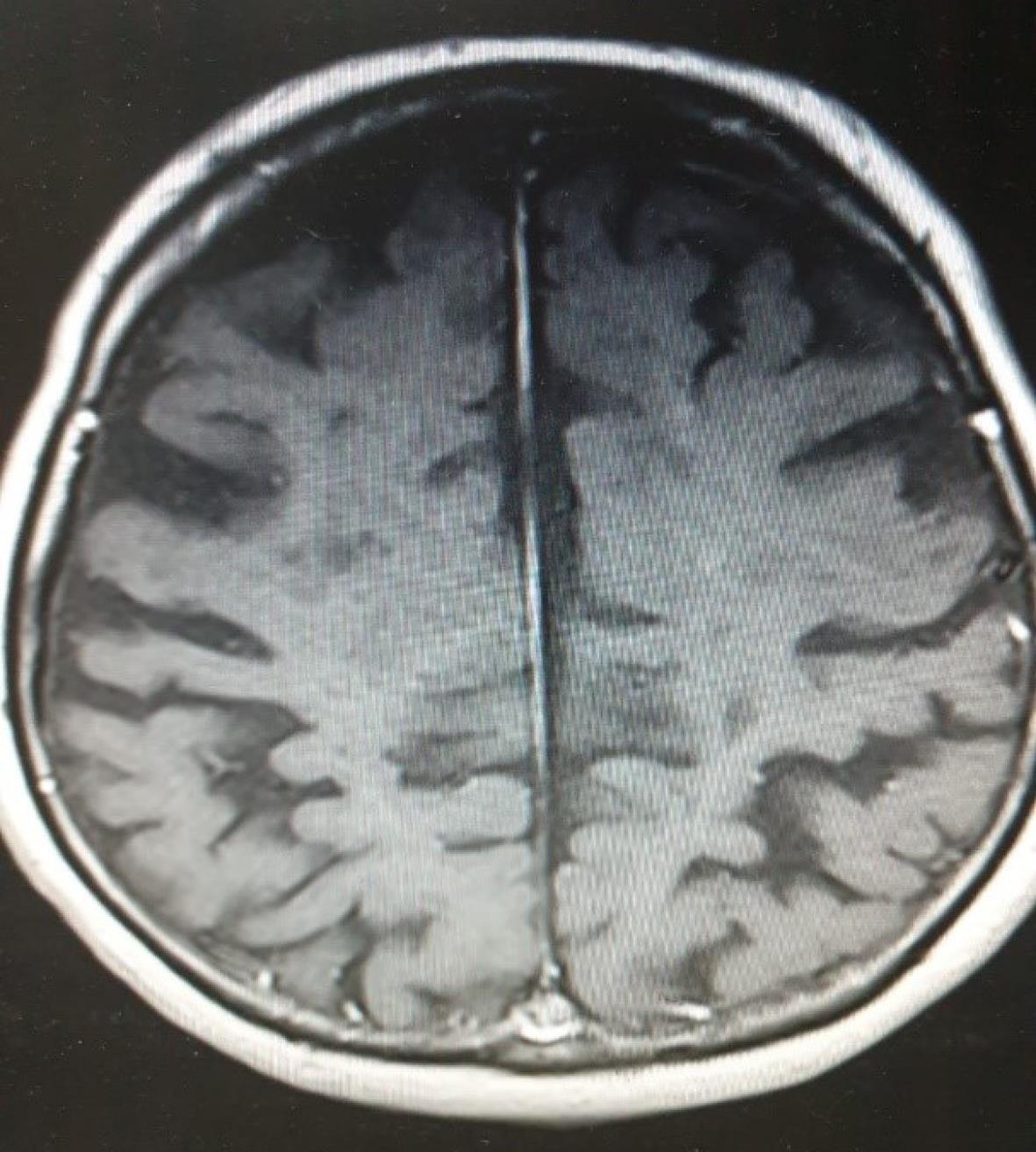
Figure 1.
Faint Enhancement on the Magnetic Resonance Imaging
.
Faint Enhancement on the Magnetic Resonance Imaging
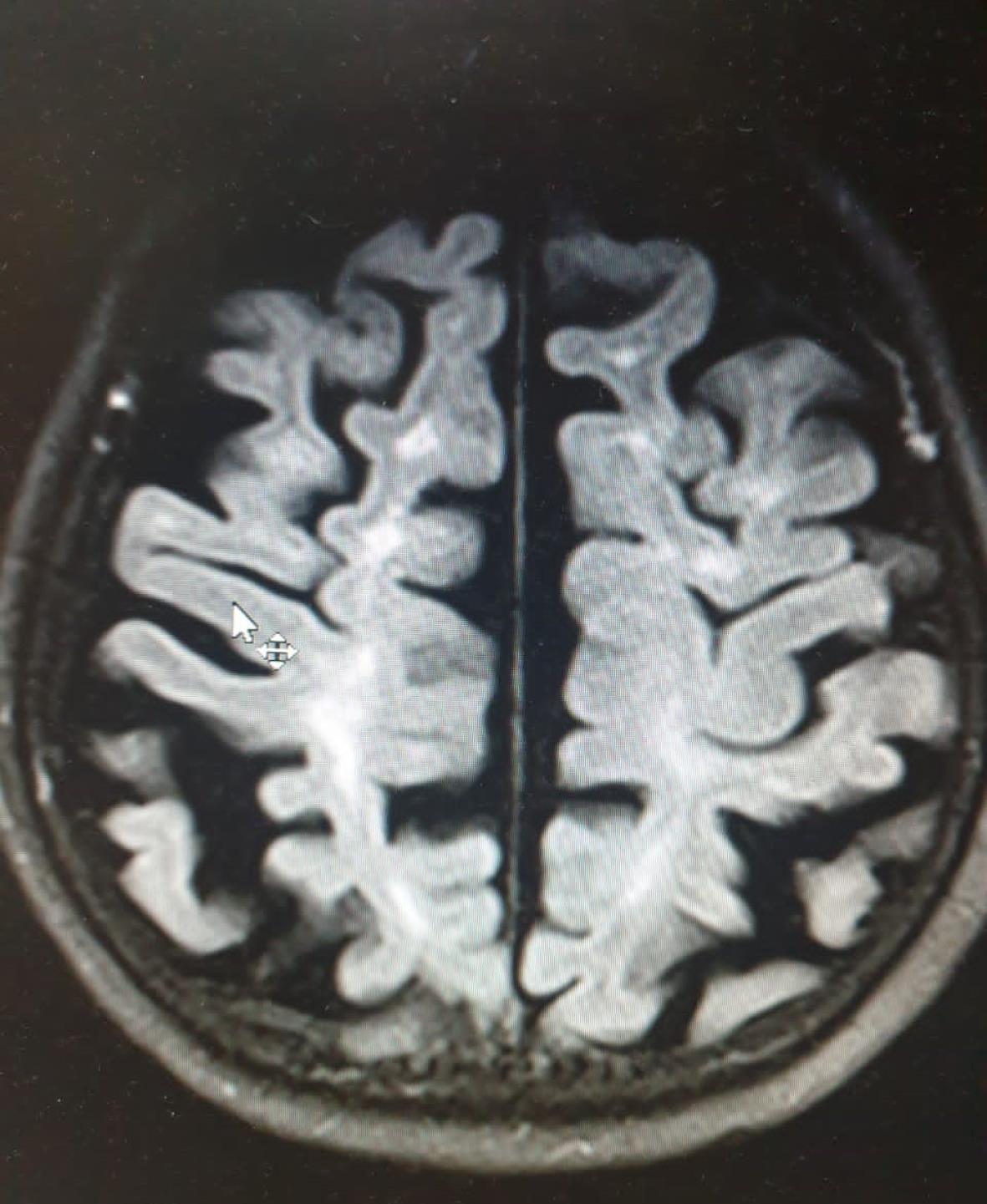
Figure 2.
Cortical Plaques and Cortical Atrophy on the Magnetic RESONANCE IMAGING
.
Cortical Plaques and Cortical Atrophy on the Magnetic RESONANCE IMAGING
The patient denied any previous history of seizures or intake of antiepileptic medications. She was ultimately treated with anti-epileptic agents (Levetiracetam), and her EDSS returned to the baseline level once she had received an intravenous injection of methylprednisolone with a dose of 5 g for five days. Furthermore, there was not any epileptic event in follow-ups after discharge.
Discussion
MS is a chronic inflammatory demyelinating disorder of the CNS leading to neurodegeneration.6 Patients with MS have a higher risk of developing epileptic seizures. The link between MS subtypes and the severity of epilepsy and the effect of disease-modifying treatments on how much a patient might become afflicted with seizures is not entirely understood yet.7 The risk of developing epilepsy in MS patients is three times higher than that in the general population8,9; however, the incidence of epileptic seizures in these patients was determined to be 0.5% to 8.3% in different studies.10,11
According to a recent meta-analysis, the incidence of epilepsy might be positively correlated with EDSS8; hence, patients with MS who later develop epilepsy are at greater risk of mortality.12 It is recognized that epileptic seizures in MS are linked to more widespread inflammatory cortical lesions compared to MS patients without epilepsy.13
In a clinical trial on patients between 18 and 55 years of age by Kappos et al, two patients (0.5%) experienced epilepsy after fingolimod treatment.14 In another placebo-controlled trial on adults by Calabresi et al, three patients (1%) in the fingolimod group reported convulsions.15 Furthermore, Cohen et al did not observe any cases of new-onset epilepsy in their cohort of MS patients under treatment with fingolimod.16 Nevertheless, convulsions did occur in 6 pediatric patients (5.6%), as reported by Chitnis et al in 2018.17 It can be concluded that convulsions are more common in children than in adults after treatment with fingolimod.
As a sphingosine 1-phosphate inhibitor, fingolimod prevents lymphocytes from entering CNS,18,19 and its discontinuation has lately been recognized as a risk factor that might predispose MS patients to rare but serious events, a condition requiring immediate and appropriate action to prevent progression and unfavorable outcomes.20 With a half-life of 6 days, fingolimod attains a steady baseline concentration in the blood after 1-2 months of intake, negatively regulating the population of circulating lymphocytes only within hours from its first dosage. However, the effect is completely reversed within 6–8 weeks after discontinuation of the drug,21 which is generally secondary to pregnancy and lactation, ongoing progression, and adverse effects.22 Symptoms associated with withdrawal may also occur after four weeks to four months from cessation.23
In the case of our patient, a decision was made to cease fingolimod therapy due to her ongoing progression. Despite the relapse of the disease, the patient demonstrated an epileptic attack in the second week from the discontinuation of fingolimod.
Seizure is more prevalent in MS patients compared to the average population. Although it can be a manifestation of relapsing disease,24 Paudel et al reported antiepileptic effects for fingolimod,25 implying that such effects could be easily lifted upon discontinuing fingolimod therapy. Withdrawal can be associated with regions of contrast enhancements on brain MRI,26 as witnessed in our patient who exhibited fair degrees of enhancement.
The disease-modifying anti-epileptic benefits of fingolimod have been well-established in experimental studies. When fingolimod was given to pentylenetetrazol-induced kindling mice, there were fewer seizures and fewer bursts of activity, as measured by electroencephalography.27 Fingolimod was also shown to have anti-epileptic effects in a genetically modified murine model of epilepsy. Early long-term therapy (ELTT) had a substantial anti-epileptogenic impact, as indicated by a decrease in seizure duration and frequency compared to the control group.28
The location of S1P1-immunostained neurons and astrocytes in the dentate gyrus and cornu ammonis 3 (CA3) of the hippocampus following intraperitoneal kainic acid (KA) delivery in mice suggests that S1P1 signaling may contribute to astrocyte proliferation secondary to KA-induced toxicity.29 Inhibiting S1P1-induced signaling with fingolimod was found to diminish reactive astrogliosis in the rat model of status epilepticus (SE), as confirmed with fewer glial fibrillary acidic protein positive cells in the hippocampus area.30 Given the documented role of neuroinflammation in epileptogenesis31 and fingolimod’s potential to reduce neuroinflammation, altering the S1P pathway could be a potential therapeutic option to counteract epileptogenesis.32
Although mechanisms underlying epileptogenesis are unknown, there is mounting evidence that neuroinflammation plays a role.33,34 Fingolimod therapy was reported to dramatically lower hippocampus expression of tumor necrosis factor-alpha (TNF-α) and interleukin-1 (IL-1) in a rat SE model compared to the control group because fingolimod prevented neuroinflammation during epileptogenesis, which reduced neuronal hyperexcitability in the chronic epileptic condition. The activity of astrocytes and microglia also characterizes neuroinflammation.35,36 In experimental rats, fingolimod therapy was demonstrated to attenuate the hippocampus activation of astrocytes and microglia after SE caused by Lithium-Pilocarpine injection.35 Moreover, the anti-inflammatory effect of fingolimod was confirmed upon downregulation of hippocampus NF-κB activity in rodents.37 The report of infection and inflammation of the COVID-19 contributing to seizures and cerebrovascular accidents also confirms the role of neuroinflammation in this regard.38
Suggested to be linked with synaptic function, the mTOR signaling pathway is thought to influence neuronal excitability, leading to epileptogenesis.39 This finding indicates that the modulation of the mTOR signaling pathway might be an effective intervention in several kinds of epilepsy.40,41 In a genetically induced murine model of epilepsy, fingolimod was found to confer similar beneficial effects. ELTT with fingolimod was shown to repress mTOR signaling activity temporarily. However, after the fingolimod therapy was stopped (after five months), the mTOR axis activity returned to normal, along with the emergence of seizures. These data imply that the anti-epileptic effects of fingolimod could actually emanate from its inhibitory effects on the mTOR signaling pathway.28
Conclusion
According to these findings, it is believed that the fits of seizure in our case might have been caused secondary to the termination of the anti-epileptogenic and anti-inflammatory effects of fingolimod. Therefore, the discontinuation of this medication warrants close monitoring and follow-up of patients.
Ethics statement
This study was conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Disclosure of funding source
The authors received no financial or any type of support for the research, authorship, and/or publication of this article.
Conflict of interests declaration
The authors declare that they have no conflict of interests.
Acknowledgements
In this study, the authors would like to express their gratitude to the patient and her family who consented to participate in the study. It is confirmed that this work was not subject to institutional review board approval.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, [ Reza Mosaddeghi Heris].
Consent for publication
The mentioned patient has verbally given her consent for the publication of this case report, and written consent will be obtained and sent if necessary.
References
- Compston A, Coles A. Multiple sclerosis. Lancet 2008; 372(9648):1502-17. doi: 10.1016/s0140-6736(08)61620-7 [Crossref] [ Google Scholar]
- Tremlett H, Zhao Y, Rieckmann P, Hutchinson M. New perspectives in the natural history of multiple sclerosis. Neurology 2010; 74(24):2004-15. doi: 10.1212/WNL.0b013e3181e3973f [Crossref] [ Google Scholar]
- Fazekas F, Bajenaru O, Berger T, Fabjan TH, Ledinek AH, Jakab G. How does fingolimod (Gilenya®) fit in the treatment algorithm for highly active relapsing-remitting multiple sclerosis?. Front Neurol 2013; 4:10. doi: 10.3389/fneur.2013.00010 [Crossref] [ Google Scholar]
- Mehling M, Johnson TA, Antel J, Kappos L, Bar-Or A. Clinical immunology of the sphingosine 1-phosphate receptor modulator fingolimod (FTY720) in multiple sclerosis. Neurology 2011; 76(8 Suppl 3):S20-7. doi: 10.1212/WNL.0b013e31820db341 [Crossref] [ Google Scholar]
- Huwiler A, Zangemeister-Wittke U. The sphingosine 1-phosphate receptor modulator fingolimod as a therapeutic agent: recent findings and new perspectives. Pharmacol Ther 2018; 185:34-49. doi: 10.1016/j.pharmthera.2017.11.001 [Crossref] [ Google Scholar]
- Volpi C, Orabona C, Macchiarulo A, Bianchi R, Puccetti P, Grohmann U. Preclinical discovery and development of fingolimod for the treatment of multiple sclerosis. Expert Opin Drug Discov 2019; 14(11):1199-212. doi: 10.1080/17460441.2019.1646244 [Crossref] [ Google Scholar]
- Pack A. Is there a relationship between multiple sclerosis and epilepsy? If so what does it tell us about epileptogenesis?. Epilepsy Curr 2018; 18(2):95-6. doi: 10.5698/1535-7597.18.2.95 [Crossref] [ Google Scholar]
- Gasparini S, Ferlazzo E, Ascoli M, Sueri C, Cianci V, Russo C. Risk factors for unprovoked epileptic seizures in multiple sclerosis: a systematic review and meta-analysis. Neurol Sci 2017; 38(3):399-406. doi: 10.1007/s10072-016-2803-7 [Crossref] [ Google Scholar]
- Marrie RA, Reider N, Cohen J, Trojano M, Sorensen PS, Cutter G. A systematic review of the incidence and prevalence of sleep disorders and seizure disorders in multiple sclerosis. Mult Scler 2015; 21(3):342-9. doi: 10.1177/1352458514564486 [Crossref] [ Google Scholar]
- Koch M, Uyttenboogaart M, Polman S, De Keyser J. Seizures in multiple sclerosis. Epilepsia 2008; 49(6):948-53. doi: 10.1111/j.1528-1167.2008.01565.x [Crossref] [ Google Scholar]
- Poser CM, Brinar VV. Epilepsy and multiple sclerosis. Epilepsy Behav 2003; 4(1):6-12. doi: 10.1016/s1525-5050(02)00646-7 [Crossref] [ Google Scholar]
- Chou IJ, Kuo CF, Tanasescu R, Tench CR, Tiley CG, Constantinescu CS. Epilepsy and associated mortality in patients with multiple sclerosis. Eur J Neurol 2019; 26(2):342-e23. doi: 10.1111/ene.13821 [Crossref] [ Google Scholar]
- Calabrese M, De Stefano N, Atzori M, Bernardi V, Mattisi I, Barachino L. Extensive cortical inflammation is associated with epilepsy in multiple sclerosis. J Neurol 2008; 255(4):581-6. doi: 10.1007/s00415-008-0752-7 [Crossref] [ Google Scholar]
- Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010; 362(5):387-401. doi: 10.1056/NEJMoa0909494 [Crossref] [ Google Scholar]
- Calabresi PA, Radue EW, Goodin D, Jeffery D, Rammohan KW, Reder AT. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13(6):545-56. doi: 10.1016/s1474-4422(14)70049-3 [Crossref] [ Google Scholar]
- Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010; 362(5):402-15. doi: 10.1056/NEJMoa0907839 [Crossref] [ Google Scholar]
- Chitnis T, Arnold DL, Banwell B, Brück W, Ghezzi A, Giovannoni G. Trial of fingolimod versus interferon beta-1a in pediatric multiple sclerosis. N Engl J Med 2018; 379(11):1017-27. doi: 10.1056/NEJMoa1800149 [Crossref] [ Google Scholar]
- Kappos L, O’Connor P, Radue EW, Polman C, Hohlfeld R, Selmaj K. Long-term effects of fingolimod in multiple sclerosis: the randomized FREEDOMS extension trial. Neurology 2015; 84(15):1582-91. doi: 10.1212/wnl.0000000000001462 [Crossref] [ Google Scholar]
- Mehling M, Brinkmann V, Antel J, Bar-Or A, Goebels N, Vedrine C. FTY720 therapy exerts differential effects on T cell subsets in multiple sclerosis. Neurology 2008; 71(16):1261-7. doi: 10.1212/01.wnl.0000327609.57688.ea [Crossref] [ Google Scholar]
- Hatcher SE, Waubant E, Nourbakhsh B, Crabtree-Hartman E, Graves JS. Rebound syndrome in patients with multiple sclerosis after cessation of fingolimod treatment. JAMA Neurol 2016; 73(7):790-4. doi: 10.1001/jamaneurol.2016.0826 [Crossref] [ Google Scholar]
- Massberg S, von Andrian UH. Fingolimod and sphingosine-1-phosphate--modifiers of lymphocyte migration. N Engl J Med 2006; 355(11):1088-91. doi: 10.1056/NEJMp068159 [Crossref] [ Google Scholar]
- Sorensen PS, Koch-Henriksen N, Petersen T, Ravnborg M, Oturai A, Sellebjerg F. Recurrence or rebound of clinical relapses after discontinuation of natalizumab therapy in highly active MS patients. J Neurol 2014; 261(6):1170-7. doi: 10.1007/s00415-014-7325-8 [Crossref] [ Google Scholar]
- Ghadiri M, Fitz-Gerald L, Rezk A, Li R, Nyirenda M, Haegert D. Reconstitution of the peripheral immune repertoire following withdrawal of fingolimod. Mult Scler 2017; 23(9):1225-32. doi: 10.1177/1352458517713147 [Crossref] [ Google Scholar]
- Langenbruch L, Krämer J, Güler S, Möddel G, Geßner S, Melzer N. Seizures and epilepsy in multiple sclerosis: epidemiology and prognosis in a large tertiary referral center. J Neurol 2019; 266(7):1789-95. doi: 10.1007/s00415-019-09332-x [Crossref] [ Google Scholar]
- Paudel YN, Angelopoulou E, Piperi C, Gnatkovsky V, Othman I, Shaikh MF. From the molecular mechanism to pre-clinical results: anti-epileptic effects of fingolimod. Curr Neuropharmacol 2020; 18(11):1126-37. doi: 10.2174/1570159x18666200420125017 [Crossref] [ Google Scholar]
- Sato K, Niino M, Kawashima A, Yamada M, Miyazaki Y, Fukazawa T. Disease exacerbation after the cessation of fingolimod treatment in Japanese patients with multiple sclerosis. Intern Med 2018; 57(18):2647-55. doi: 10.2169/internalmedicine.0793-18 [Crossref] [ Google Scholar]
- Gol M, Ghorbanian D, Hassanzadeh S, Javan M, Mirnajafi-Zadeh J, Ghasemi-Kasman M. Fingolimod enhances myelin repair of hippocampus in pentylenetetrazol-induced kindling model. Eur J Pharm Sci 2017; 96:72-83. doi: 10.1016/j.ejps.2016.09.016 [Crossref] [ Google Scholar]
- Leo A, Citraro R, Amodio N, De Sarro C, Gallo Cantafio ME, Constanti A. Fingolimod exerts only temporary antiepileptogenic effects but longer-lasting positive effects on behavior in the WAG/Rij rat absence epilepsy model. Neurotherapeutics 2017; 14(4):1134-47. doi: 10.1007/s13311-017-0550-y [Crossref] [ Google Scholar]
- Lee DH, Jeon BT, Jeong EA, Kim JS, Cho YW, Kim HJ. Altered expression of sphingosine kinase 1 and sphingosine-1-phosphate receptor 1 in mouse hippocampus after kainic acid treatment. Biochem Biophys Res Commun 2010; 393(3):476-80. doi: 10.1016/j.bbrc.2010.02.027 [Crossref] [ Google Scholar]
- Zhu XD, Chen JS, Zhou F, Liu QC, Chen G, Zhang JM. Relationship between plasma high mobility group box-1 protein levels and clinical outcomes of aneurysmal subarachnoid hemorrhage. J Neuroinflammation 2012; 9:194. doi: 10.1186/1742-2094-9-194 [Crossref] [ Google Scholar]
- Terrone G, Balosso S, Pauletti A, Ravizza T, Vezzani A. Inflammation and reactive oxygen species as disease modifiers in epilepsy. Neuropharmacology 2020; 167:107742. doi: 10.1016/j.neuropharm.2019.107742 [Crossref] [ Google Scholar]
- Cipriani R, Chara JC, Rodríguez-Antigüedad A, Matute C. FTY720 attenuates excitotoxicity and neuroinflammation. J Neuroinflammation 2015; 12:86. doi: 10.1186/s12974-015-0308-6 [Crossref] [ Google Scholar]
- Paudel YN, Shaikh MF, Shah S, Kumari Y, Othman I. Role of inflammation in epilepsy and neurobehavioral comorbidities: implication for therapy. Eur J Pharmacol 2018; 837:145-55. doi: 10.1016/j.ejphar.2018.08.020 [Crossref] [ Google Scholar]
- Vezzani A, Balosso S, Ravizza T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat Rev Neurol 2019; 15(8):459-72. doi: 10.1038/s41582-019-0217-x [Crossref] [ Google Scholar]
- Gao F, Liu Y, Li X, Wang Y, Wei D, Jiang W. Fingolimod (FTY720) inhibits neuroinflammation and attenuates spontaneous convulsions in lithium-pilocarpine induced status epilepticus in rat model. Pharmacol Biochem Behav 2012; 103(2):187-96. doi: 10.1016/j.pbb.2012.08.025 [Crossref] [ Google Scholar]
- Devinsky O, Vezzani A, Najjar S, De Lanerolle NC, Rogawski MA. Glia and epilepsy: excitability and inflammation. Trends Neurosci 2013; 36(3):174-84. doi: 10.1016/j.tins.2012.11.008 [Crossref] [ Google Scholar]
- Gao F, Gao Y, Meng F, Yang C, Fu J, Li Y. The sphingosine 1-phosphate analogue FTY720 alleviates seizure-induced overexpression of P-glycoprotein in rat hippocampus. Basic Clin Pharmacol Toxicol 2018; 123(1):14-20. doi: 10.1111/bcpt.12973 [Crossref] [ Google Scholar]
- Karimi H, Sarmadian R, Gilani A, Salajegheh P, Nejad Biglari H, Gholizadeh M. Cerebrovascular accident in a child with precursor B-cell acute lymphoblastic leukemia and coronavirus disease 2019: a case report. J Med Case Rep 2022; 16(1):452. doi: 10.1186/s13256-022-03672-5 [Crossref] [ Google Scholar]
- Meng XF, Yu JT, Song JH, Chi S, Tan L. Role of the mTOR signaling pathway in epilepsy. J Neurol Sci 2013; 332(1-2):4-15. doi: 10.1016/j.jns.2013.05.029 [Crossref] [ Google Scholar]
- Galanopoulou AS, Gorter JA, Cepeda C. Finding a better drug for epilepsy: the mTOR pathway as an antiepileptogenic target. Epilepsia 2012; 53(7):1119-30. doi: 10.1111/j.1528-1167.2012.03506.x [Crossref] [ Google Scholar]
- Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci 2009; 29(21):6964-72. doi: 10.1523/jneurosci.0066-09.2009 [Crossref] [ Google Scholar]