Int J Drug Res Clin. 2023;1:e24.
doi: 10.34172/ijdrc.2023.e24
Review Article
Effects of Tibial Nerve Electrical Stimulation on Bowel Dysfunction in Multiple Sclerosis: A Systematic Review
Fateme Tahmasbi 1, 2  , Hanieh Salehi-Pourmehr 1, 3
, Hanieh Salehi-Pourmehr 1, 3  , Sakineh Hajebrahimi 1
, Sakineh Hajebrahimi 1  , Farzin Soleimanzadeh 1, *
, Farzin Soleimanzadeh 1, * 
Author information:
1Research Center for Evidence-Based Medicine, Iranian EBM Centre: A JBI Centre of Excellence, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
2Social Determinants of Health Research Center, Health Management and Safety Promotion Research Institute, Tabriz University of Medical Sciences, Tabriz, Iran
3Medical Philosophy and History Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Background:
This study set out to conduct a thorough review of the effects of electrical tibial nerve stimulation in multiple sclerosis (MS) patients with bowel dysfunction.
Methods:
Several medical databases were searched comprehensively from inception to September 2023. Studies with available English full texts and results for bowel dysfunction following percutaneous tibial nerve stimulation (PTNS) or transcutaneous tibial nerve stimulation (TTNS) were considered. The publications that were included underwent a comprehensive assessment by two independent reviewers, and pertinent data were retrieved. The quality of the included studies was evaluated using Cochrane guidelines.
Results:
From a total of 200 studies, only 2 prospective interventional studies were eligible for entering this systematic review with no control arm. A total of 93 patients diagnosed with MS (44 females and 49 males) with a median age of 48.32 years were included. Both studies applied PTNS in 30-minute sessions for a maximum of 12 weeks and reported an overall improvement following PTNS in MS-associated bowel dysfunction.
Conclusion:
This systematic review demonstrated that PTNS can be an effective way of minimizing fecal incontinence in MS patients. However, due to the highly limited number of available publications on this issue, we cannot generalize these findings to larger populations.
Keywords: Multiple sclerosis, Tibial nerve stimulation, Fecal incontinence, Functional constipation, Systematic review
Copyright and License Information
© 2023 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Multiple sclerosis (MS) is a chronic, inflammatory, and autoimmune disease that affects approximately 400 000 and 2.3 million individuals in the United States and around the globe, respectively. 1 Bowel dysfunctions are one of the most common complications among MS patients, which are most frequently seen as functional constipation (FC) and fecal incontinence (FI), leading to severely compromised life quality in addition to social integration and academic concerns.2 Bowel dysfunction occurs approximately in two-thirds of patients,3 and its incidence is seemingly correlated to the severity and duration of the disease.4,5 However, no adequate level of evidence is currently available for the management of bowel dysfunction in MS patients.6
The underlying pathophysiology of MS is an autoimmune formation of demyelinating plaques in neurological pathways that produce a wide spectrum of sensory and motor lesions, resulting in various neurological manifestations that can cause disabilities with different severity.7,8
Given that MS is the most prevalent cause of non-traumatic neurological impairment in young people and considering the estimated onset age of 20 to 50, minimizing its adverse effects has always been a clinical and scholarly concern.9,10 Furthermore, since there is an absence of a definite cure for the disease, managing the symptoms and improving the life quality of patients are of critical importance, regardless of the course and the prognosis.
Electrical tibial nerve stimulation (TNS), a neuromodulation technique that includes stimulating the tibial nerve with electricity, alleviates urinary and erectile dysfunction by modulating the sacral roots. This method has been suggested as a promising, non-invasive, and safe method to elevate neurologically challenged conditions, including MS.11,12 With slight changes in technique, some methods fall under this category, including transcutaneous tibial nerve stimulation (TTNS) and percutaneous tibial nerve stimulation (PTNS). However, they are both based on delivering electrical impulses to the tibial nerve.
Although there exists a considerable amount of evidence on how the urinary function of MS patients can be improved through TNS,13-16 unfortunately, limited knowledge is available regarding the defecatory function. Therefore, we conducted a systematic review of the current literature in an attempt to address the lack of evidence on the topic and to draw attention to the existing knowledge gap.
Methods
The PRISMA guidelines were followed during the course of this study to guarantee thorough data reporting,17 and the study protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO: CRD42022319030).
Search Strategy
Studies pertinent to the topic were looked up in electronic medical databases, including MEDLINE (via PubMed), Scopus, Embase, and Web of Science from inception to September 2023. The search strategy was formulated based on the PICO: Patients/Population (P): MS patients, Intervention (I): Electrical stimulation of the tibial nerve, Comparison (C): Not specified, and Outcome (O): Fecal incontinence (Table 1). The full search strategy is presented in Supplementary file 1.
Table 1.
Different Key Terms and Combinations Used in Designing the Search Strategy
|
Population
|
Intervention
|
Outcome
|
| MS |
Tibial nerve stimulation |
Fecal incontinence |
| Encephalomyelitis |
Posterior tibial nerve stimulation |
Feces incontinence |
| Encephalomyelitis disseminate |
Percutaneous tibial nerve stimulation |
Bowel dysfunction |
| MS |
Transcutaneous tibial nerve stimulation |
Gas incontinence |
|
|
Transcutaneous electrical nerve stimulation |
Stool incontinence |
|
|
Percutaneous electrical nerve stimulation |
Fecal leak* |
|
|
Tibial nerve transdermal stimulation |
Stool leak* |
|
|
Neuromodulation |
|
|
|
Transdermal electrostimulation |
|
Note. MS: Multiple sclerosis.
The OR Boolean operator was used between the terms in each column, while AND was used to combine the columns
Inclusion and Exclusion Criteria
Studies that met the inclusion criteria were conducted on MS patients, used any form of TNS, including PTNS or TTNS as the intervention during the experiment, and had an available English full text. Studies that were conducted on other conditions, used other forms of intervention, or did not have an available English full text were excluded.
Literature Screening and Data Extraction
For further investigation, Rayyan was used to integrate the findings from the literature search.18 Two independent reviewers investigated the titles and the abstracts of the studies, and those passing the initial survey were screened based on their full text. At each level, any discrepancies were discussed between the two reviewers, and if an agreement could not be achieved, a third reviewer was brought in. The data extracted from the selected studies included bibliographic information (e.g., author, origin, and year of publication), study design, intervention method, participants’ characteristics (e.g., age, number, course, and duration of the disease), intervention data (e.g., type, duration, and follow up); and study outcomes.
Quality Assessment
The Cochrane scale, which is thoroughly explained in the Cochrane Handbook for Systematic Reviews of Interventions V.5.1.0, was used to assess the trials’ level of quality and risk of bias.19 Seven types of bias were taken into consideration, including random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and staff (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and any other biases. Furthermore, each study would receive one of three quality ratings: good, medium, or low if it met the criteria of 5–7, 3–4, or 0–2 categories, respectively. This phase was separately completed by two reviewers, and in the event of a dispute, a third reviewer was consulted.
Analysis
Conducting meta-analysis was not an option since studies were too limited and heterogeneous to be comparable. Hence, the narrative method was used to report the findings.
Results
Search and Selection
A total of 200 studies were retrieved from the electronic search of databases. Deduplication was conducted using Rayyan, leaving 106 articles for initial screening. A secondary search was done for the included literature and references of relevant reviews to prevent leaving out any citations. After a full-text review, only two studies were found to be eligible for entering this systematic review.20,21 This process is demonstrated in Figure 1.
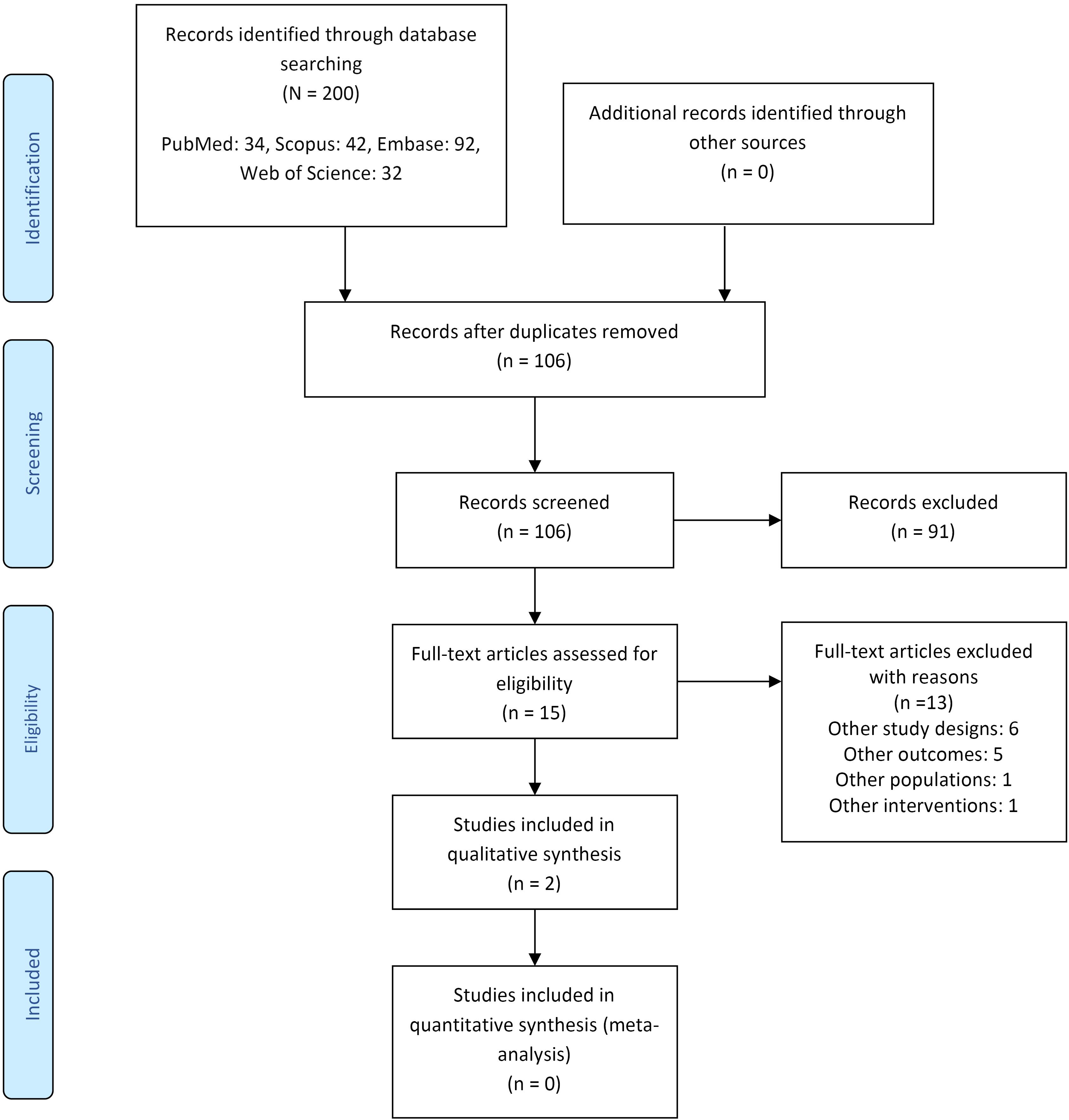
Figure 1.
The PRISMA Flow Diagram
.
The PRISMA Flow Diagram
Characteristics of Participants
A total of 93 patients diagnosed with MS were enrolled consisting of 44 females and 49 males. The median age of the participants was 48.32 years, and the median duration of the MS disease was 14.77 years. Forty-six patients had relapsing-remitting MS (RRMS) courses, 35 secondary progressive MS (SPMS), and 11 primary-progressive MS (PPMS). One study was undertaken in the United Kingdom21 and the other in Switzerland.20 Both citations were prospective studies with no control group and one arm of MS patients. Regarding the duration of intervention, both studies applied PTNS in 30-minute sessions for a maximum of 12 weeks. The difference was that one study used an initial three-week trial and then continued this trial in responsive patients for 12 weeks.21 Furthermore, both studies used PTNS as their method of intervention. One study employed the Rome III criteria, and the Cleveland Clinic Fecal Incontinence Score (CCFIS) to evaluate bowel dysfunction,20 while the other utilized the Wexner incontinence score.21 Table 2 provides further informationregarding the characteristics of the included studies.
Table 2.
Characteristics of the Included Studies
|
Author
|
Year
|
Origin
|
Population
|
Patients’ Condition
|
Symptoms
|
Measurement
|
Main Results
|
|
No.
|
Age
|
Sex (F/M)
|
Course
|
MS Severity
|
Duration
(y)
|
| Sacco et al20 |
2020 |
Switzerland |
60 |
48.5 |
19/41 |
RRMS: 24 SPMS: 27 PPMS: 8 |
EDSS: 4.5 |
15.2 (9.8–23.0) |
FI + FC: 25
FI: 5
FC: 30 |
CCFIS, The Rome III criteria |
PTNS significantly improves FI and FC symptoms in MS patients |
| Sanagapalli et al21 |
2018 |
UK |
33 |
48 |
25/8 |
RRMS: 22 SPMS: 8
PPMS: 3 |
|
10 (6 -13) |
FI: 33 |
Wexner incontinence score, The Rockwood score, Two visual analogue scales, The Bristol Stool Form Scale |
Responder patients were more symptomatic at baseline and had greater improvements in bowel symptom scores and quality of life. |
Note. MS: Multiple sclerosis; RRMS: Relapsing-remitting multiple sclerosis; SPMS: Secondary-progressive multiple sclerosis; PPMS: Primary-progressive multiple sclerosis; EDSS: Expanded disability status scale; FI: Fecal incontinence; FC: Functional constipation; CCFIS: Cleveland clinic fecal incontinence score; PTNS: Percutaneous tibial nerve stimulation.
Quality of Included Studies
Figures 2 and 3 illustrate the findings of the quality assessment. Both of the articles were considered to be high risk in terms of selection, performance, and detection bias, low risk in terms of attrition bias, and unclear risk in reporting and other biases.
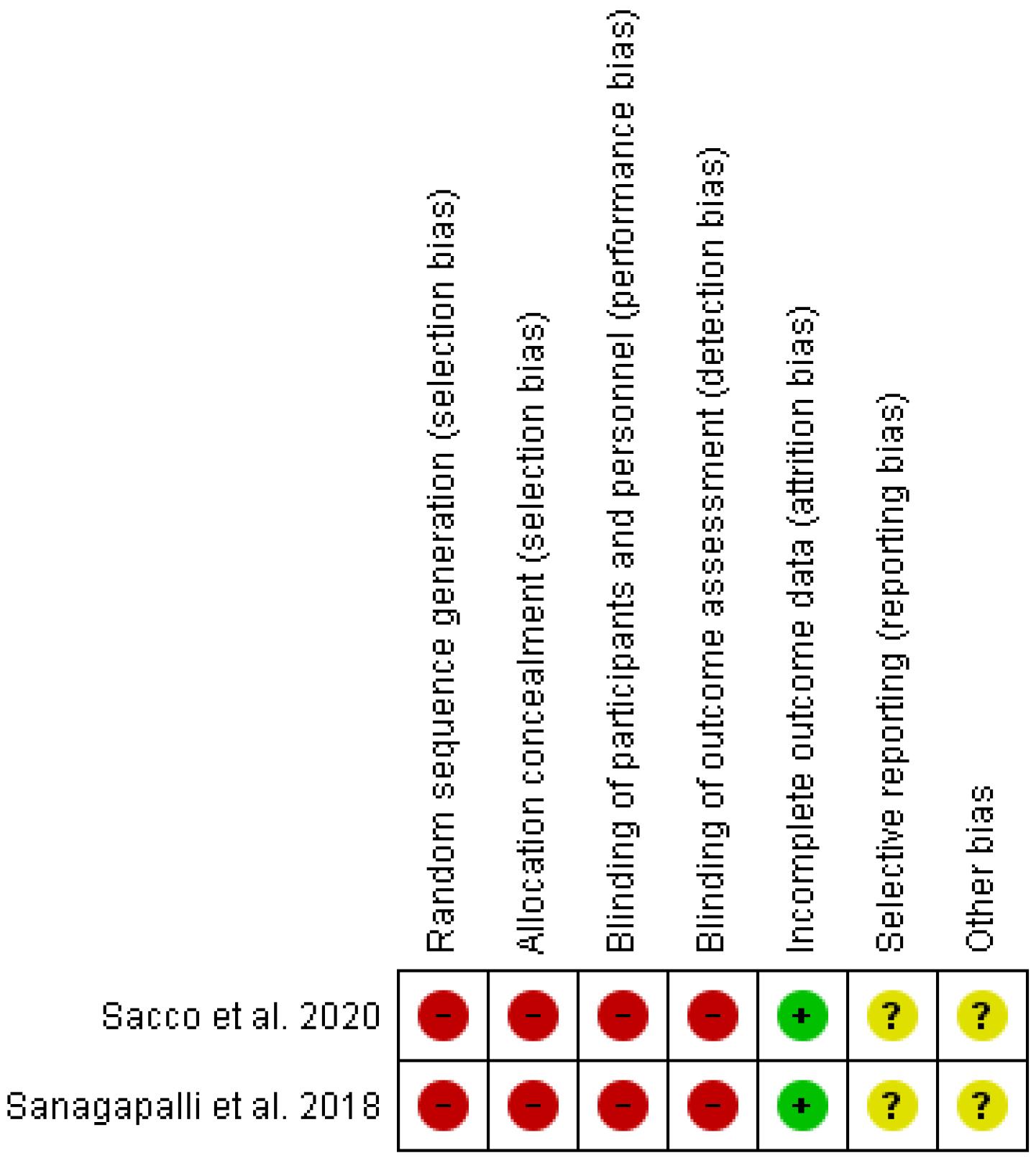
Figure 2.
Risk of Bias Summary: Authors’ Judgements about Each Risk of Bias Item for Each Included Study
.
Risk of Bias Summary: Authors’ Judgements about Each Risk of Bias Item for Each Included Study
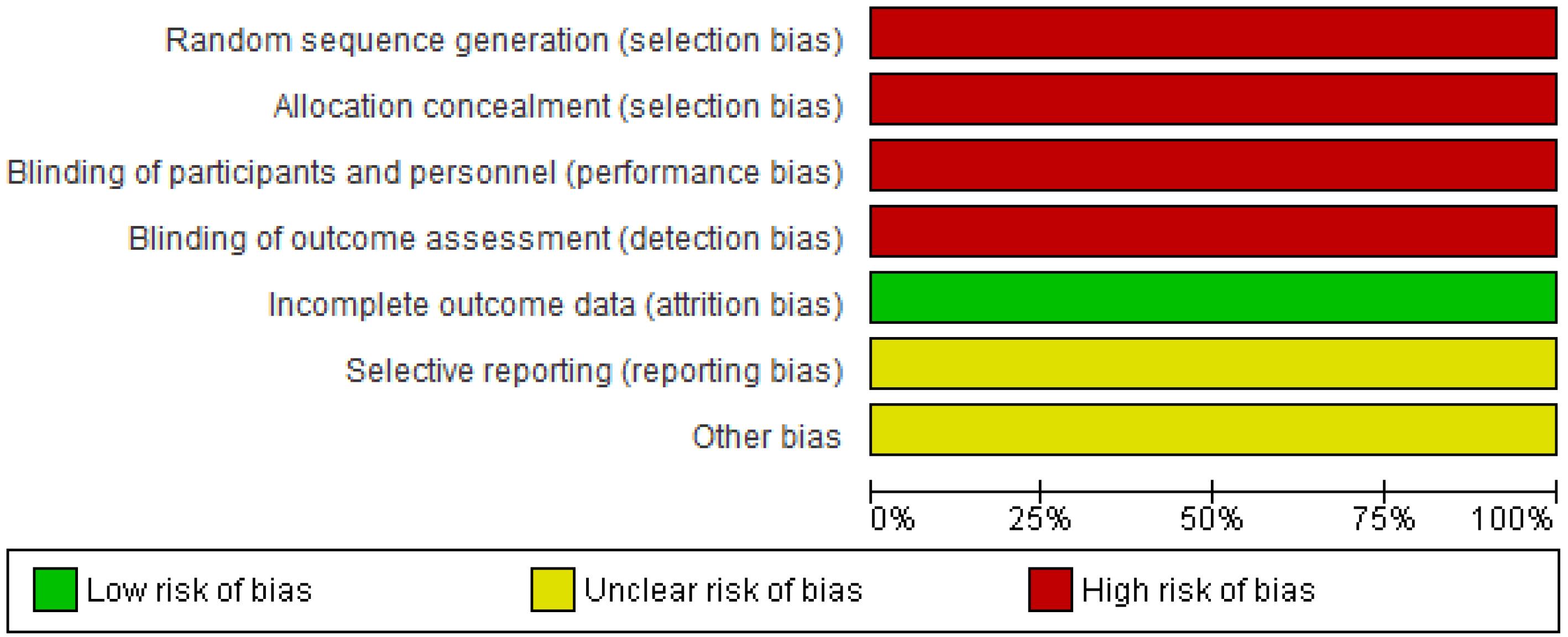
Figure 3.
Risk of Bias Graph: Authors’ Judgements about Each Risk of Bias Item Presented as Percentages across All Included Studies
.
Risk of Bias Graph: Authors’ Judgements about Each Risk of Bias Item Presented as Percentages across All Included Studies
Discussion
Patients with MS frequently experience bowel dysfunction, which has a serious negative effect on their quality of life.22 Bowel issues are reported by more than half of MS patients, according to studies, and they are linked to lower quality of life ratings. A study conducted in Norway examined the prevalence of gastrointestinal dysfunction among MS patients aged 2-5 years following diagnosis.22
Bowel dysfunction caused by neurological diseases such as MS is referred to as neurogenic bowel dysfunction (NBD).23 The management of NBD in MS patients involves obtaining a careful bowel history and assessing bowel function before neurological symptoms appear. Optimizing nutrition and laxative use to establish a useful and regular bowel routine is part of the conservative therapy of NBD. Transanal irrigation has been found to lessen NBD symptoms and enhance quality of life in cases where conservative therapy is insufficient. Furthermore, surgical options are available for patients who do not respond to other interventions.23
Uncertainty surrounds the pathophysiology of intestinal dysfunction in MS patients. Overall, the underlying mechanism of bowel dysfunction among MS patients is related to the neurological damage caused by the disease. MS is characterized by demyelination and inflammation in the central nervous system, which can affect the nerves that control bowel function. Constipation, FI, and reduced rectal sensation are just a few of the gastrointestinal issues that can result from this interruption in nerve transmission.24
Different parameters can affect the patient’s symptom load, including the type of MS, duration, and severity of the disease, in addition to variables such as psychological dysfunction, patients’ activity, and pharmacological therapies.25-28
The overall results of this systematic review suggested that the percutaneous stimulation of the tibial nerve improves bowel dysfunction in MS patients. Despite the limited number of included studies, the current study suggested that PTNS can have positive effects on the management of bowel dysfunction symptoms in MS patients. A response rate of 56.7% was found in Sacco and colleagues’ research for treating FI in MS patients, and there was a slight but statistically significant decline in the number of patients who reported still having FC at the end of the 12-week PTNS therapy period. In more precise terms, 7 out of 55 patients achieved FC freedom, whereas patients who had FC negative status at baseline had no evidence of FC at the end of the 12-week intervention.20 After 12 weeks of PTNS treatment, Sanagapalli et al observed a greater responder rate (79%) in MS patients with FI and a substantial reduction in incontinence symptoms.21
To our knowledge, these two studies are the only available publications on how TNS can affect bowel dysfunction in MS patients. Therefore, additional supporting data is unavailable. However, several studies have reported noteworthy results in patients with other etiologies of bowel dysfunction. For instance, in a prospective observational-interventional study by Dedemadi and Takano on 22 patients with etiologies, including previous surgery, obstetric, or idiopathic the number of FI episodes significantly decreased from 4.7 to 1.5, a decreased Wexner score from 10.2 to 6.9, following the administration of bilateral TTNS in 30-minute session twice a week for 6 weeks.29 Hotouras and colleagues’ cohort study on 150 patients with etiologies, including obstetric, surgical, radiotherapy, trauma, and idiopathic showed a statistically significant CCFI score from 12.0 ± 3.9 to 9.4 ± 4.6, following the final maintenance therapy session and 12 PTNS sessions.30 This pattern of improvement is supported by several studies as well.31-33
The precise mechanism of PTNS and how it acts as a neuromodulator is not completely defined yet. However, several suggestions have been made so far. For example, it has been suggested to directly modulate the peripheral nerve roots that innervate the pelvic floor through the same spinal roots or to stimulate cortical pontine activity.34-36 This modulation can improve the contraction of the rectum by several outcomes: by enhancing the blood flow of the rectum due to an elevated stimulation of autonomous fibers37 and by causing a reduction in anal canal stiffness.38 However, none of these suggestions can thoroughly justify the result across the studies; therefore, the mechanism of action in TNS should be investigated more comprehensively in the future.
It is also noteworthy to mention a recent umbrella review that was conducted to evaluate the clinical effects of posterior tibial nerve stimulation on FI. The findings of 14 systematic reviews indicated a general improvement in a number of study characteristics such as bowel habits and quality of life. The summary results revealed no statistically significant changes in FI when PTNS was compared with sham or sacral nerve stimulation (P> 0.05). On the other hand, FI episodes were considerably lower in the PTNS arm, and PTNS caused fewer FI episodes than sham, according to the subgroup analysis of the kind of intervention in the control group. Therefore, based on the findings of trials with a small population, PTNS may be beneficial to patients with FI.39
Limitations
Even though the information in our evaluation is the most recent one, it was subject to several limitations. The first and the most obvious was the limited number of studies which led to extremely restricted and ungeneralizable findings. Second, the absence of a control arm in both of the included studies is another obvious source of bias. In addition, the included studies recruited a different number of patients regarding the MS course. This means that in Sacco and colleagues’ study, 40% of FI patients were RRMS, but this rate was 67% in Sanapagalli’s study21, which can be associated with different responses to the treatment. Moreover, the long-term effects of a 12-week PTNS treatment were not covered by any of the investigations. Hence, caution must be taken in interpreting these findings due to the limited number of available publications on the topic.
Conclusion
In conclusion, caution must be taken in interoperating the findings of the current clinical studies, and the potential effectiveness of PTNS for treating gastrointestinal dysfunction in MS patients needs to be established by additional research. More high-quality research, namely, those that ideally incorporate a control arm can help produce more generalizable conclusions.
Ethics statement
Not applicable.
Disclosure of funding source
This study was supported by the Deputy of Research in Tabriz University of Medical Sciences (Grant code: 69762).
Conflict of interests declaration
The authors report no conflict of interests.
Acknowledgments
The research protocol was approved by the Research Center for Evidence-based Medicine, Iranian EBM Centre: A JBI Centre of Excellence, Faculty of Medicine, Tabriz University of Medical Sciences. Also, we thank the Clinical Research Development Unit of Tabriz Valiasr Hospital, Tabriz University of Medical Sciences, Tabriz, Iran for their assistance in this research.
Data availability statement
All data used in conduction of this review is reported in the manuscript or Supplementary file 1.
Consent for publication
Not applicable.
Supplementary files
Supplementary file 1 contains full search strategy.
(pdf)
References
- National Multiple Sclerosis Society. MS Prevalence. 2015. Available from: http://www.nationalmssociety.org/About-the-Society/MS-Prevalence.
- Dibley L, Coggrave M, McClurg D, Woodward S, Norton C. “It’s just horrible”: a qualitative study of patients’ and carers’ experiences of bowel dysfunction in multiple sclerosis. J Neurol 2017; 264(7):1354-61. doi: 10.1007/s00415-017-8527-7 [Crossref] [ Google Scholar]
- Hinds JP, Eidelman BH, Wald A. Prevalence of bowel dysfunction in multiple sclerosis A population survey. Gastroenterology 1990; 98(6):1538-42. doi: 10.1016/0016-5085(90)91087-m [Crossref] [ Google Scholar]
- Nordenbo AM, Andersen JR, Andersen JT. Disturbances of ano-rectal function in multiple sclerosis. J Neurol 1996; 243(6):445-51. doi: 10.1007/bf00900497 [Crossref] [ Google Scholar]
- Munteis E, Andreu M, Téllez MJ, Mon D, Ois A, Roquer J. Anorectal dysfunction in multiple sclerosis. Mult Scler 2006; 12(2):215-8. doi: 10.1191/135248506ms1254oa [Crossref] [ Google Scholar]
- Gulick EE. Neurogenic Bowel Dysfunction Over the Course of Multiple Sclerosis: A Review. Int J MS Care 2022; 24(5):209-217. doi: 10.7224/1537-2073.2021-007 [Crossref] [ Google Scholar]
- Wu GF, Alvarez E. The immunopathophysiology of multiple sclerosis. Neurol Clin 2011; 29(2):257-78. doi: 10.1016/j.ncl.2010.12.009 [Crossref] [ Google Scholar]
- Huang WJ, Chen WW, Zhang X. Multiple sclerosis: pathology, diagnosis and treatments. Exp Ther Med 2017; 13(6):3163-6. doi: 10.3892/etm.2017.4410 [Crossref] [ Google Scholar]
- Browne P, Chandraratna D, Angood C, Tremlett H, Baker C, Taylor BV. Atlas of multiple sclerosis 2013: a growing global problem with widespread inequity. Neurology 2014; 83(11):1022-4. doi: 10.1212/wnl.0000000000000768 [Crossref] [ Google Scholar]
- National Multiple Sclerosis Society. Who Gets MS? 2018. Available from: http://www.nationalmssociety.org/What-is-MS/Who-Gets-MS.
- Tahmasbi F, Hosseini S, Hajebrahimi S, Mosaddeghi Heris R, Salehi-Pourmehr H. Efficacy of tibial nerve stimulation in neurogenic lower urinary tract dysfunction among patients with multiple sclerosis: a systematic review and meta-analysis. Urol Res Pract 2023; 49(2):100-11. doi: 10.5152/tud.2023.22241 [Crossref] [ Google Scholar]
- Kabay SC, Yucel M, Kabay S. Acute effect of posterior tibial nerve stimulation on neurogenic detrusor overactivity in patients with multiple sclerosis: urodynamic study. Urology 2008; 71(4):641-5. doi: 10.1016/j.urology.2007.11.135 [Crossref] [ Google Scholar]
- Guitynavard F, Mirmosayyeb O, Emami Razavi SZ, Hosseini M, Hosseinabadi AM, Ghajarzadeh M. Percutaneous posterior tibial nerve stimulation (PTNS) for lower urinary tract symptoms (LUTSs) treatment in patients with multiple sclerosis (MS): a systematic review and meta-analysis. Mult Scler Relat Disord 2022; 58:103392. doi: 10.1016/j.msard.2021.103392 [Crossref] [ Google Scholar]
- Canbaz Kabay S, Kabay S, Mestan E, Cetiner M, Ayas S, Sevim M. Long term sustained therapeutic effects of percutaneous posterior tibial nerve stimulation treatment of neurogenic overactive bladder in multiple sclerosis patients: 12-months results. Neurourol Urodyn 2017; 36(1):104-10. doi: 10.1002/nau.22868 [Crossref] [ Google Scholar]
- Gobbi C, Digesu GA, Khullar V, El Neil S, Caccia G, Zecca C. Percutaneous posterior tibial nerve stimulation as an effective treatment of refractory lower urinary tract symptoms in patients with multiple sclerosis: preliminary data from a multicentre, prospective, open label trial. Mult Scler 2011; 17(12):1514-9. doi: 10.1177/1352458511414040 [Crossref] [ Google Scholar]
- Zecca C, Digesu GA, Robshaw P, Singh A, Elneil S, Gobbi C. Maintenance percutaneous posterior nerve stimulation for refractory lower urinary tract symptoms in patients with multiple sclerosis: an open label, multicenter, prospective study. J Urol 2014; 191(3):697-702. doi: 10.1016/j.juro.2013.09.036 [Crossref] [ Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010; 8(5):336-41. doi: 10.1016/j.ijsu.2010.02.007 [Crossref] [ Google Scholar]
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016; 5(1):210. doi: 10.1186/s13643-016-0384-4 [Crossref] [ Google Scholar]
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons; 2019.
- Sacco R, Braga A, Disanto G, Digesu GA, Maino P, Koetsier E. Effectiveness of percutaneous posterior tibial nerve stimulation for the management of bowel dysfunction in multiple sclerosis patients. Mult Scler 2021; 27(10):1577-84. doi: 10.1177/1352458520972267 [Crossref] [ Google Scholar]
- Sanagapalli S, Neilan L, Lo JYT, Anandan L, Liwanag J, Raeburn A. Efficacy of percutaneous posterior tibial nerve stimulation for the management of fecal incontinence in multiple sclerosis: a pilot study. Neuromodulation 2018; 21(7):682-7. doi: 10.1111/ner.12764 [Crossref] [ Google Scholar]
- Nortvedt MW, Riise T, Frugård J, Mohn J, Bakke A, Skår AB. Prevalence of bladder, bowel and sexual problems among multiple sclerosis patients two to five years after diagnosis. Mult Scler 2007; 13(1):106-12. doi: 10.1177/1352458506071210 [Crossref] [ Google Scholar]
- Emmanuel A. Neurogenic bowel dysfunction. F1000Res 2019;8. 10.12688/f1000research.20529.1.
- Preziosi G, Gordon-Dixon A, Emmanuel A. Neurogenic bowel dysfunction in patients with multiple sclerosis: prevalence, impact, and management strategies. Degener Neurol Neuromuscul Dis 2018; 8:79-90. doi: 10.2147/dnnd.s138835 [Crossref] [ Google Scholar]
- Munteis E, Andreu M, Téllez MJ, Mon D, Ois A, Roquer J. Anorectal dysfunction in multiple sclerosis. Mult Scler 2006; 12(2):215-8. doi: 10.1191/135248506ms1254oa [Crossref] [ Google Scholar]
- Wiesel PH, Norton C, Glickman S, Kamm MA. Pathophysiology and management of bowel dysfunction in multiple sclerosis. Eur J Gastroenterol Hepatol 2001; 13(4):441-8. doi: 10.1097/00042737-200104000-00025 [Crossref] [ Google Scholar]
- Glick ME, Meshkinpour H, Haldeman S, Bhatia NN, Bradley WE. Colonic dysfunction in multiple sclerosis. Gastroenterology 1982; 83(5):1002-7. [ Google Scholar]
- Coggrave M, Norton C, Cody JD. Management of faecal incontinence and constipation in adults with central neurological diseases. Cochrane Database Syst Rev 2014; 2014(1):CD002115. doi: 10.1002/14651858.CD002115.pub5 [Crossref] [ Google Scholar]
- Dedemadi G, Takano S. Efficacy of bilateral transcutaneous posterior tibial nerve stimulation for fecal incontinence. Perm J 2018; 22:17-231. doi: 10.7812/tpp/17-231 [Crossref] [ Google Scholar]
- Hotouras A, Murphy J, Walsh U, Allison M, Curry A, Williams NS. Outcome of percutaneous tibial nerve stimulation (PTNS) for fecal incontinence: a prospective cohort study. Ann Surg 2014; 259(5):939-43. doi: 10.1097/SLA.0b013e3182a6266c [Crossref] [ Google Scholar]
- Hotouras A, Thaha MA, Allison ME, Currie A, Scott SM, Chan CL. Percutaneous tibial nerve stimulation (PTNS) in females with faecal incontinence: the impact of sphincter morphology and rectal sensation on the clinical outcome. Int J Colorectal Dis 2012; 27(7):927-30. doi: 10.1007/s00384-011-1405-3 [Crossref] [ Google Scholar]
- Peña Ros E, Parra Baños PA, Benavides Buleje JA, Muñoz Camarena JM, Escamilla Segade C, Candel Arenas MF. Short-term outcome of percutaneous posterior tibial nerve stimulation (PTNS) for the treatment of faecal incontinence. Tech Coloproctol 2016; 20(1):19-24. doi: 10.1007/s10151-015-1380-8 [Crossref] [ Google Scholar]
- Edenfield AL, Amundsen CL, Wu JM, Levin PJ, Siddiqui NY. Posterior tibial nerve stimulation for the treatment of fecal incontinence: a systematic evidence review. Obstet Gynecol Surv 2015; 70(5):329-41. doi: 10.1097/ogx.0000000000000171 [Crossref] [ Google Scholar]
- Vandoninck V, van Balken MR, Finazzi Agrò E, Petta F, Micali F, Heesakkers JP. Percutaneous tibial nerve stimulation in the treatment of overactive bladder: urodynamic data. Neurourol Urodyn 2003; 22(3):227-32. doi: 10.1002/nau.10111 [Crossref] [ Google Scholar]
- Shafik A, Ahmed I, El-Sibai O, Mostafa RM. Percutaneous peripheral neuromodulation in the treatment of fecal incontinence. Eur Surg Res 2003; 35(2):103-7. doi: 10.1159/000069399 [Crossref] [ Google Scholar]
- Wexner SD. Percutaneous tibial nerve stimulation in faecal incontinence. Lancet 2015; 386(10004):1605-6. doi: 10.1016/s0140-6736(15)60508-6 [Crossref] [ Google Scholar]
- Emmanuel AV, Kamm MA. Laser Doppler measurement of rectal mucosal blood flow. Gut 1999; 45(1):64-9. doi: 10.1136/gut.45.1.64 [Crossref] [ Google Scholar]
- Vaizey CJ, Kamm MA, Turner IC, Nicholls RJ, Woloszko J. Effects of short term sacral nerve stimulation on anal and rectal function in patients with anal incontinence. Gut 1999; 44(3):407-12. doi: 10.1136/gut.44.3.407 [Crossref] [ Google Scholar]
- Tahmasbi F, Mosaddeghi-Heris R, Soleimanzadeh F, Ghaderpanah R, Sadrian S, Hajebrahimi S, et al. Effects of posterior tibial nerve stimulation on fecal incontinence: an umbrella review. Neuromodulation. 2023. 10.1016/j.neurom.2023.06.004.