Int J Drug Res Clin. 2023;1:e15.
doi: 10.34172/ijdrc.2023.e15
Original Article
Does Cranberry Supplementation Decrease Noninvasive Fibrosis Scores in NAFLD Patients? A Randomized Clinical Trial
Kourosh Masnadi Shirazi 1  , Elham Shirinpour 2, Arman Masnadi Shirazi 2, Zeinab Nikniaz 1, *
, Elham Shirinpour 2, Arman Masnadi Shirazi 2, Zeinab Nikniaz 1, * 
Author information:
1Liver and Gastrointestinal Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Background:
Due to the lack of effective treatment for non-alcoholic fatty liver disease (NAFLD), we assumed that cranberry supplementation may be effective in these patients. Therefore, we investigated the effect of cranberry supplementation on fibrosis levels in patients with NAFLD.
Methods:
This trial was designed as a randomized controlled clinical trial. It included 110 adult patients (aged>18 years) with NAFLD. All patients were visited by an expert dietitian and received the hypocaloric diet plus vitamin E supplement. Then, the patients entered into a six-month trial to receive cranberry capsules (55 patients) or placebo (55 patients). We calculated the NAFLD fibrosis score (NFS), fibrosis scores based on 4 factors (FIB-4), and aspartate aminotransferase (AST) to platelet ratio index (APRI). The intention-to-treat (ITT) approach was used to analyze the data.
Results:
The participants’ mean age was 43.16±10.23 years. The demographic and baseline clinical features were similar in the two groups. In the cranberry group, there were significant changes in APRI (P=0.03), and NFS (P<0.001) scores. In the placebo groups, there were significant changes in APRI (P=0.005), FIB-4 (P=0.03), and NFS (P<0.001) scores. However, no between-group significant differences were observed in the changes in FIB-4 (P=0.64), APRI (P=0.78), and NFS score (P=0.38).
Conclusion:
Based on the results, cranberry supplementation was not more effective than placebo in liver fibrosis grade.
Keywords: Vaccinium macrocarpon, Fibrosis scores, Non-alcoholic fatty liver disease
Copyright and License Information
© 2023 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is described by the storage of extra fats in the liver after excluding the secondary causes of fat accumulation.1 The global prevalence of NAFLD was 25.24%. The increase in the prevalence of NAFLD was more significant in Asian countries which is in line with the changes in lifestyle parameters in these countries.2 This disease affects morbidity, mortality, and healthcare utilization.3,4
Different treatment methods, including lifestyle modification (diet and exercise interventions), bariatric surgery, and drug interventions have been used in the treatment of NAFLD.5 However, the results of these treatments were not promising, and considering the invasive nature of some of these approaches, other complications may arise. Hence, researchers are focusing on the effect of new supplementary herbal treatments in these patients.6 One of the new herbal treatments that exhibited favorable results in animal models was cranberry (Vaccinium macrocarpon).7,8 Our previous reports indicated that cranberry has a promising impact on weight and lipid profile in NAFLD patients.9 In addition to cardiometabolic effects, we hypothesized that cranberry may also have positive effects on steatosis in NAFLD patients. Cranberry is a rich source of different polyphenols10 such as stilbins and anthocyanins. Various animal investigations demonstrated the positive effect of these polyphenols on steatosis levels. In a clinical trial, Hormoznejad et al reported that the fibrosis grade is significantly lower in NAFLD patients who received cranberry supplementation compared with placebo.11
Owing to the high prevalence and burden of NAFLD and due to the lack of effective treatment for this disease, we assumed that lifestyle modification accompanied by cranberry supplementation may be effective in decreasing the steatosis stage in patients with NAFLD. Considering the scarcity of studies in this field, the present clinical trial was conducted to assess the effect of cranberry supplementation on fibrosis levels in patients with NAFLD.
Methods
This trial was designed as a parallel-arm randomized controlled clinical trial. We included the adult patients aged > 18 years with NAFLD. The NAFLD was diagnosed by expert gastroenterologists using liver enzyme level and ultrasonography results. We excluded pregnant or breastfeeding patients, patients with endocrine and other hepatic disorders, cardiovascular disease, urinary disease, and lung failure, alcoholic beverage drinkers, and patients who took dietary supplements other than vitamin E.
The result of the previous study was used to calculate the sample size.12 In this regard, we used the G-Power software and assumed a type I error of 5% and a type II error of 20%. The sample size was calculated as 37 per group. Considering that some patients may drop out of the study, 55 patients were enrolled.
A total of 110 patients participated in this investigation. A researcher who did not have any role in the research created a random list using the GraphPad QuickCalcs program and concealed the list until the end of the investigation. All patients were visited by an expert dietitian and received the hypocaloric diet plus vitamin E supplement. In addition, 55 patients received cranberry capsules (intervention group), and 55 patients received the placebo (control group) for six months. We instructed the patients to take the capsules with a meal, and we had a pre-planned telephone call to assess the patient’s compliance with the intervention and ask about any issues that arose. The patients were visited monthly, and their compliance with interventions and lifestyle modifications was checked.
Shari Company in Iran provided the Cranberry and placebo capsules. According to company information, the active ingredient of the cranberry capsule is Vaccinium macrocarpon (144 mg). The placebo did not contain this active ingredient. A colleague who did not have any role in other parts of the trial labeled supplements as A and B. The participants, the endpoint evaluator, and the researcher who analyzed data were blind to group assignment.
Evaluation of the Therapeutic Efficacy
To assess therapeutic efficacy, we calculated the NAFLD fibrosis score (NFS), fibrosis scores based on 4 factors (FIB-4), and aspartate aminotransferase (AST) to platelet ratio index (APRI). For the calculation of these indices, we need to measure fasting liver enzymes and platelet levels using the colorimetric method (Parsazmoun, Tehran, Iran).
FIB-4 was calculated using a standard equation.13 FIB-4 < 1.45 was considered the probability of no advanced fibrosis, 1.45–3.25 was considered the indeterminate level of fibrosis, and > 3.25 was considered the probability of advanced fibrosis.
The APRI was calculated using a standard formula.14 The APRI score of less than 0.5 indicated no fibrosis, 0.5-1.5 indicated moderate fibrosis and higher than 1.5 indicated significant fibrosis.
Furthermore, the NFS was calculated using the standard formula.15 The score < -1.45 showed no fibrosis, -1.45 to 0.67 indicated intermediate fibrosis, and > 0.67 suggested advanced fibrosis.
Statistical Analysis
For data analysis, the 16th version of SPSS was applied, and the data were analyzed based on the intention to treat analysis. The Kolmogorov-Smirnov test was applied to test if the set of data comes from a normal distribution. The continuous variables were reported as mean and standard deviations (SD), and nominal and categorical variables were reported as frequency and percent (%). A comparison of the continuous variables before and after supplementations was conducted using a paired sample t test. Between-group comparisons were conducted using the chi-square test and independent t test. The one-way analysis of covariance (ANCOVA) analysis was conducted to compare the post-intervention values between groups adjusted for age, gender, body mass index, and pre-intervention levels and a P value of less than 0.05 was considered significant.
Results
As depicted in Figure 1, nine patients and seven patients were lost to follow-up from the intervention and placebo groups, respectively.
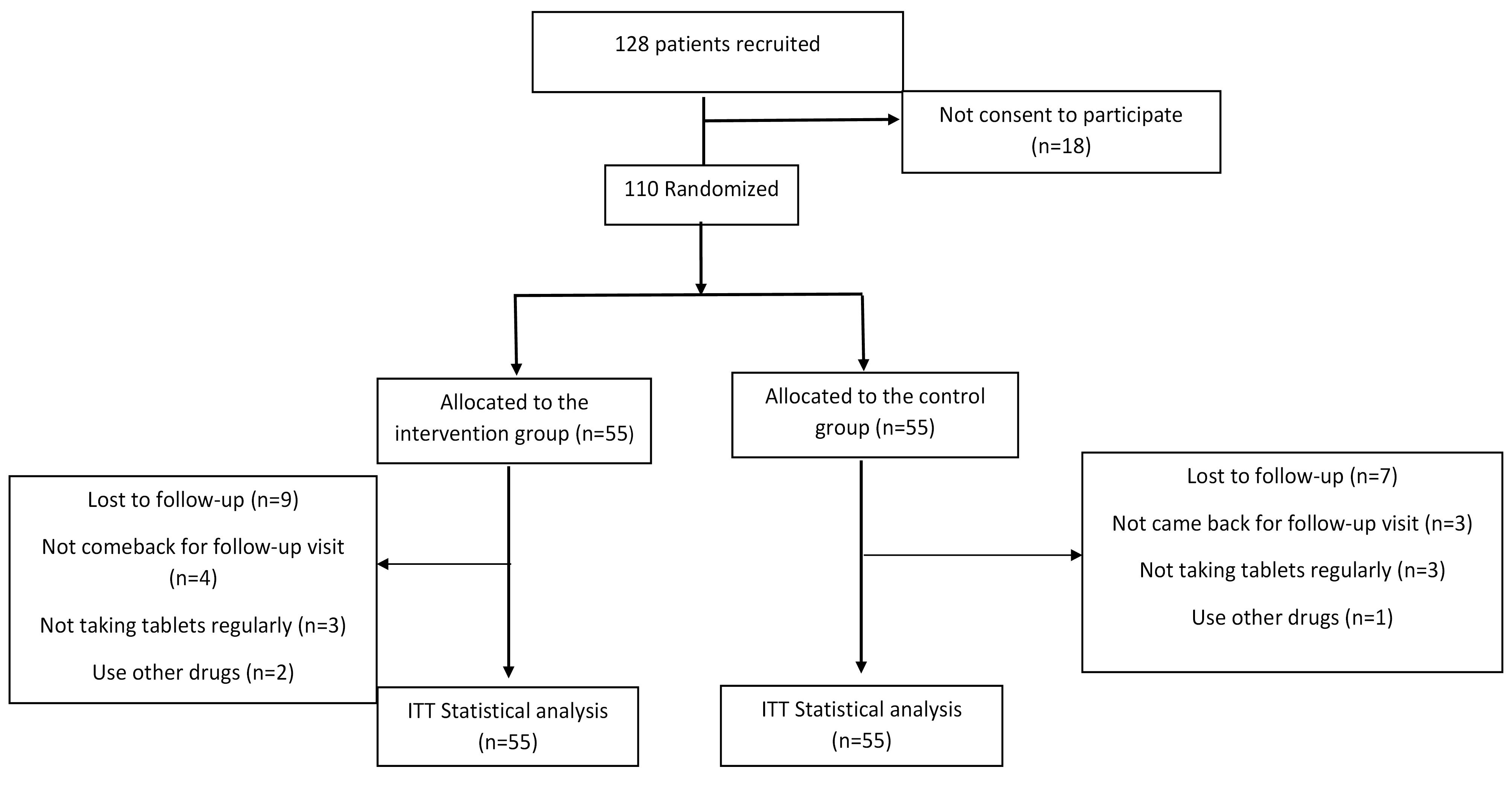
Figure 1.
Flow Chart of Patients’ Recruitment and Analysis. Note. ITT: Intension to treat
.
Flow Chart of Patients’ Recruitment and Analysis. Note. ITT: Intension to treat
The mean patients’ age was 43.16 ± 10.23 years, and 48.2% of them were male. As presented in Table 1, the baseline features were statistically similar between groups.
Table 1.
The Baseline Characteristics of Participants
|
Variables
|
Cranberry Group (n=55)
|
Placebo Group (n=55)
|
P
Value *
|
| Age (y) |
44.36 ± 10.97 |
43.64 ± 10.46 |
0.72 |
| Gender (male/female), No. (%) |
24 (43.6)/31 (56.4) |
28 (50.9)/27 (49.1) |
0.44 |
| BMI (kg/m2) |
28.21 ± 4.56 |
28.6 ± 3.87 |
0.63 |
| Platelet/L |
266.73 ± 68.40 |
265.62 ± 69.88 |
0.93 |
| ALT (IU/L) |
43.13 ± 13.76 |
47.18 ± 17.14 |
0.21 |
| AST (IU/L) |
37.54 ± 12.36 |
40.92 ± 15.58 |
0.17 |
| APRI |
0.38 ± 0.17 |
0.41 ± 0.19 |
0.37 |
| APRI < 0.5 |
76.1 |
72.9 |
0.72 |
| APRI 0.5-1.5 |
23.9 |
27.1 |
| APRI > 1.5 |
0 |
0 |
| NFS |
-2.97 ± 1.16 |
-2.93 ± 1.02 |
0.86 |
| NFS < -1.45 |
82.6 |
91.7 |
0.18 |
| NFS -1.45-0.67 |
17.3 |
8.3 |
| NFS > 0.67 |
0 |
0 |
| FIB-4 |
1.01 ± 0.46 |
1.01 ± 1.28 |
0.66 |
| FIB-4 < 1.45 |
82.6 |
87.5 |
0.50 |
| FIB-4 1.45-3.25 |
17.4 |
12.5 |
| FIB-4 > 3.25 |
0 |
0 |
Note. ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; NAFLD: Nonalcoholic fatty liver disease; NFS: NAFLD fibrosis score; FIB-4: fibrosis index based on 4 factors, APRI: Aspartate aminotransferase to platelet ratio index.
* P value of independent t-test; ** P value of chi-square test.
In terms of fibrosis scores, significant changes were observed in the intervention group in APRI (P = 0.03) and NFS (P < 0.001) scores. Likewise, significant changes were observed in the placebo groups in APRI (P = 0.005), FIB-4 (P = 0.03), and NFS (P < 0.001) scores. The changes in fibrosis grade are depicted in Figure 2 (A-C). As can be observed, in the cranberry group, 23%, 17.4%, and 13% of patients experienced a reduction in APRI grade, FIB-4 grade, and NFS grade, respectively. In the placebo group, 18.8%, 8.3%, and 4.2% of patients experienced a reduction in APRI grade, FIB-4 grade, and NFS grade, respectively. Moreover, no between-group significant differences were observed in FIB-4 (P = 0.64), APRI (P = 0.78), and NFS scores (P = 0.38).
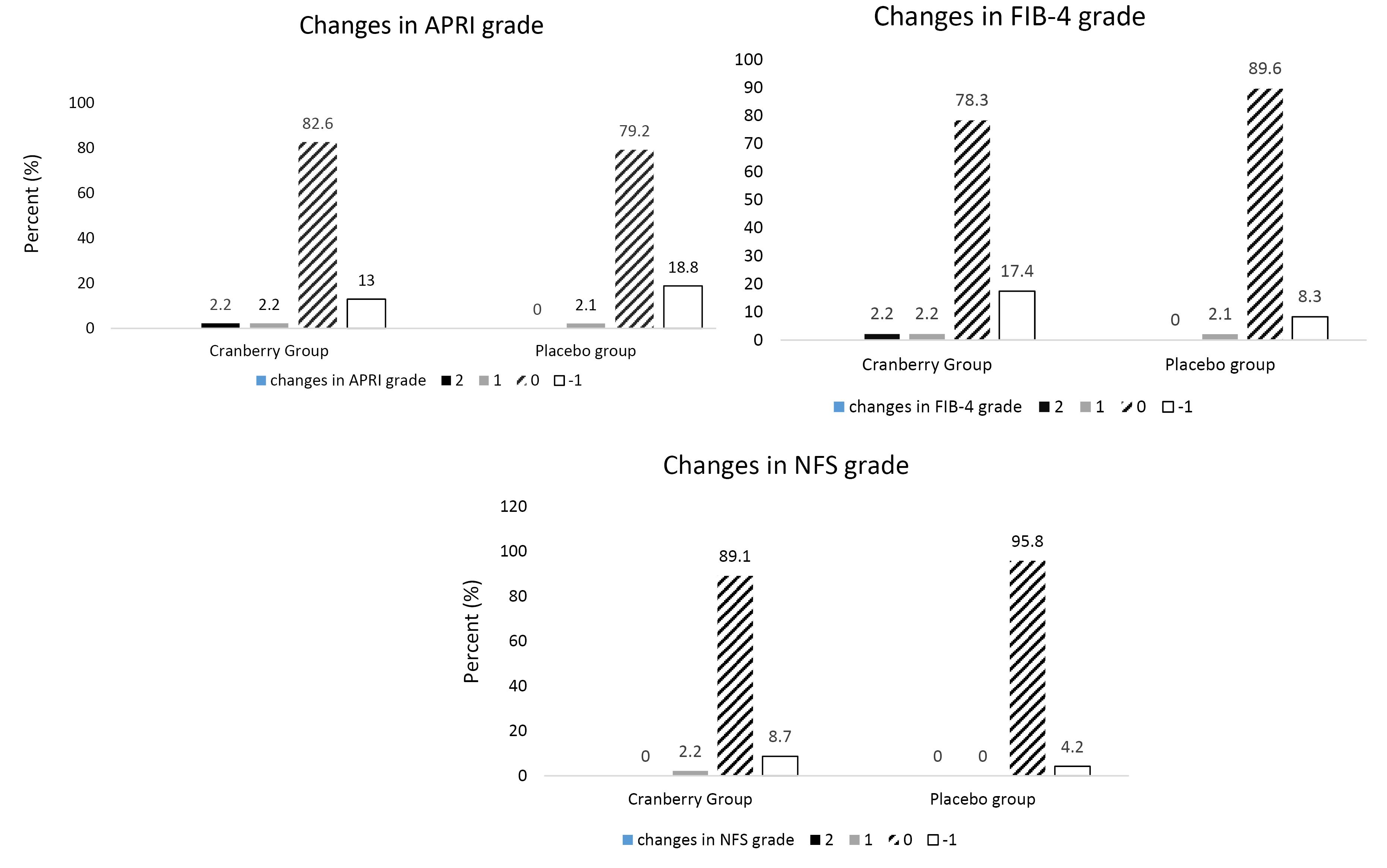

Figure 2.
(A) The Changes in APRI Score in Cranberry and Placebo Groups. Note. P value of difference between groups in 1-grade increase in APRI grade: 0.97; P-value of difference between groups in 2-grade increase in APRI grade: 0.30; P value of difference between groups in no changes in APRI grade: 0.91; P value of difference between groups in 1-grade decrease in APRI grade: 0.86. (B) The Changes in FIB-4 Score in Cranberry and Placebo Groups. Note. P value of difference between groups in 1-grade increase in FIB-4 grade: 0.97; P-value of difference between groups in 2-grade increase in FIB-4 grade: 0.30; P value of difference between groups in no changes in FIB-4 grade: 0.32; P value of difference between groups in 1-grade decrease in FIB-4 grade: 0.45. (C) The Changes in NFS Score in Cranberry and Placebo Groups. Note. P-value of difference between groups in increase in NFS grade: 0.30; P-value of difference between groups in no changes in NFS grade: 0.21; P-value of difference between groups in 1-grade decrease in NFS grade: 0.36. Abbreviations: APRI: Aspartate aminotransferase (AST) to platelet ratio index; FIB-4: Fibrosis index based on 4 factors; NAFLD: Nonalcoholic fatty liver disease; NFS: NAFLD fibrosis score
.
(A) The Changes in APRI Score in Cranberry and Placebo Groups. Note. P value of difference between groups in 1-grade increase in APRI grade: 0.97; P-value of difference between groups in 2-grade increase in APRI grade: 0.30; P value of difference between groups in no changes in APRI grade: 0.91; P value of difference between groups in 1-grade decrease in APRI grade: 0.86. (B) The Changes in FIB-4 Score in Cranberry and Placebo Groups. Note. P value of difference between groups in 1-grade increase in FIB-4 grade: 0.97; P-value of difference between groups in 2-grade increase in FIB-4 grade: 0.30; P value of difference between groups in no changes in FIB-4 grade: 0.32; P value of difference between groups in 1-grade decrease in FIB-4 grade: 0.45. (C) The Changes in NFS Score in Cranberry and Placebo Groups. Note. P-value of difference between groups in increase in NFS grade: 0.30; P-value of difference between groups in no changes in NFS grade: 0.21; P-value of difference between groups in 1-grade decrease in NFS grade: 0.36. Abbreviations: APRI: Aspartate aminotransferase (AST) to platelet ratio index; FIB-4: Fibrosis index based on 4 factors; NAFLD: Nonalcoholic fatty liver disease; NFS: NAFLD fibrosis score
Discussion
This interventional study found that although there were significant changes in fibrosis scores in both placebo and intervention groups, the between-group differences were not statistically significant. A previous study also showed the nonsignificant effect of cranberry capsules on liver fibrosis.11 However, studies in animal models have reported the favorable impact of cranberry on liver fibrosis.16
The significant changes in steatosis grade in the cranberry group can be attributed to the PPAR-α activation. As previously shown, the cranberry is a good source of the stilbenoid. In an animal study, it has been indicated that stilbenoid activates PPAR-α and consequently increases oxidation of fatty acid and decreases liver level of triglyceride.17 In addition, cranberry is a good source of anthocyanins. Sun et al showed that anthocyanins isolated from blueberry improve liver fibrosis by decreasing oxidative stress, inflammatory factors, and the activation of stellate cells in rats.18 In another animal study, Romualdo et al also detected the significant effect of anthocyanin-rich extract on liver fibrosis levels.19
This trial had the following limitations. It did not use the gold standard (histological findings) to determine the fibrosis grade in the patients. However, considering the invasive nature of this procedure, FIB-4, APRI, and NFS scores were used for monitoring NAFLD.
Conclusion
According to findings, cranberry supplementation along with lifestyle modification did not have a greater effect on liver fibrosis grade compared to placebo supplementation and lifestyle modification. Nevertheless, because of the limitations of our trial, there is a need for long-term trials with larger sample sizes to confirm these results. Moreover, it is recommended that future trials use biopsy findings to assess the fibrosis level if possible.
Ethics statement
This trial received ethics approval from the Tabriz University of Medical Sciences (IR.TBZMED.REC.1399.090). The trial protocol was also registered in the Iranian registry of clinical trials (Identifier NO. IRCT20200725048200N1; first registration date: 11.8.2020).
Conflict of interests declaration
The authors declare no conflict of interests.
Acknowledgments
The authors wish to thank the Liver and Gastrointestinal Diseases Research Center, Tabriz University of Medical Sciences for financial support.
References
- McCullough AJ. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin Liver Dis 2004; 8(3):521-33. doi: 10.1016/j.cld.2004.04.004 [Crossref] [ Google Scholar]
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64(1):73-84. doi: 10.1002/hep.28431 [Crossref] [ Google Scholar]
- Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 2013; 10(11):686-90. doi: 10.1038/nrgastro.2013.171 [Crossref] [ Google Scholar]
- Rinella M, Charlton M. The globalization of nonalcoholic fatty liver disease: prevalence and impact on world health. Hepatology 2016; 64(1):19-22. doi: 10.1002/hep.28524 [Crossref] [ Google Scholar]
- Rong L, Zou J, Ran W, Qi X, Chen Y, Cui H. Advancements in the treatment of non-alcoholic fatty liver disease (NAFLD). Front Endocrinol (Lausanne) 2022; 13:1087260. doi: 10.3389/fendo.2022.1087260 [Crossref] [ Google Scholar]
- Xu Y, Guo W, Zhang C, Chen F, Tan HY, Li S. Herbal medicine in the treatment of non-alcoholic fatty liver diseases-efficacy, action mechanism, and clinical application. Front Pharmacol 2020; 11:601. doi: 10.3389/fphar.2020.00601 [Crossref] [ Google Scholar]
- Shimizu K, Ono M, Imoto A, Nagayama H, Tetsumura N, Terada T. Cranberry attenuates progression of non-alcoholic fatty liver disease induced by high-fat diet in mice. Biol Pharm Bull 2019; 42(8):1295-302. doi: 10.1248/bpb.b18-00984 [Crossref] [ Google Scholar]
- Faheem SA, Saeed NM, El-Naga RN, Ayoub IM, Azab SS. Hepatoprotective effect of cranberry nutraceutical extract in non-alcoholic fatty liver model in rats: impact on insulin resistance and Nrf-2 expression. Front Pharmacol 2020; 11:218. doi: 10.3389/fphar.2020.00218 [Crossref] [ Google Scholar]
- Masnadi Shirazi K, Shirinpour E, Masnadi Shirazi A, Nikniaz Z. Effect of cranberry supplementation on liver enzymes and cardiometabolic risk factors in patients with NAFLD: a randomized clinical trial. BMC Complement Med Ther 2021; 21(1):283. doi: 10.1186/s12906-021-03436-6 [Crossref] [ Google Scholar]
- Narwojsz A, Tańska M, Mazur B, Borowska EJ. Fruit physical features, phenolic compounds profile and inhibition activities of cranberry cultivars (Vaccinium macrocarpon) compared to wild-grown cranberry (Vaccinium oxycoccus). Plant Foods Hum Nutr 2019; 74(3):300-6. doi: 10.1007/s11130-019-00737-7 [Crossref] [ Google Scholar]
- Hormoznejad R, Mohammad Shahi M, Rahim F, Helli B, Alavinejad P, Sharhani A. Combined cranberry supplementation and weight loss diet in non-alcoholic fatty liver disease: a double-blind placebo-controlled randomized clinical trial. Int J Food Sci Nutr 2020; 71(8):991-1000. doi: 10.1080/09637486.2020.1746957 [Crossref] [ Google Scholar]
- Novotny JA, Baer DJ, Khoo C, Gebauer SK, Charron CS. Cranberry juice consumption lowers markers of cardiometabolic risk, including blood pressure and circulating C-reactive protein, triglyceride, and glucose concentrations in adults. J Nutr 2015; 145(6):1185-93. doi: 10.3945/jn.114.203190 [Crossref] [ Google Scholar]
- Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43(6):1317-25. doi: 10.1002/hep.21178 [Crossref] [ Google Scholar]
- Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003; 38(2):518-26. doi: 10.1053/jhep.2003.50346 [Crossref] [ Google Scholar]
- Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45(4):846-54. doi: 10.1002/hep.21496 [Crossref] [ Google Scholar]
- Anhê FF, Roy D, Pilon G, Dudonné S, Matamoros S, Varin TV. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp population in the gut microbiota of mice. Gut 2015; 64(6):872-83. doi: 10.1136/gutjnl-2014-307142 [Crossref] [ Google Scholar]
- Sun Q, Yue Y, Shen P, Yang JJ, Park Y. Cranberry product decreases fat accumulation in Caenorhabditis elegans. J Med Food 2016; 19(4):427-33. doi: 10.1089/jmf.2015.0133 [Crossref] [ Google Scholar]
- Sun J, Wu Y, Long C, He P, Gu J, Yang L. Anthocyanins isolated from blueberry ameliorates CCl4 induced liver fibrosis by modulation of oxidative stress, inflammation and stellate cell activation in mice. Food Chem Toxicol 2018; 120:491-9. doi: 10.1016/j.fct.2018.07.048 [Crossref] [ Google Scholar]
- Romualdo GR, de Souza IP, de Souza LV, Prata GB, Fraga-Silva TF, Sartori A. Beneficial effects of anthocyanin-rich peels of Myrtaceae fruits on chemically-induced liver fibrosis and carcinogenesis in mice. Food Res Int 2021; 139:109964. doi: 10.1016/j.foodres.2020.109964 [Crossref] [ Google Scholar]