Int J Drug Res Clin. 2023;1:e18.
doi: 10.34172/ijdrc.2023.e18
Original Article
Therapeutic Effect of Vitamin A Administration on Disease Severity in Hospitalized COVID-19 Patients: A Pilot Open-Labeled Randomized Clinical Trial
Zeinab Nikniaz 1, *  , Mohammad Hossein Somi 1, Masood Faghih Dinevari 1, Samaneh Abbasian 1
, Mohammad Hossein Somi 1, Masood Faghih Dinevari 1, Samaneh Abbasian 1
Author information:
1Liver and Gastrointestinal Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Background:
Considering the high prevalence of the COVID-19 worldwide and since there is no effective treatment for it, the employment of complementary treatments without side effects is highlighted. So, this clinical trial aimed to assess if vitamin A injection decreases disease severity in hospitalized patients with COVID-19.
Methods:
In this pilot open-labeled randomized controlled clinical trial (RCT), 18 patients were instructed to take a daily dose of intramuscular vitamin A (maximum of 14 days), and 18 patients continued their common treatment protocols. For predicting mortality risk, we used the Coronavirus Clinical Characterization Consortium (4C) score. This score predicts mortality in hospitalized patients based on demographic and physiologic parameters. Based on this score, the patients were classified into four groups from low-risk to extremely high-risk groups.
Results:
The included participants were aged 61.43±13.54 years, and 38.8% of them were female. The findings indicated no significant differences between groups regarding baseline characteristics. In the vitamin A group, the mean 4C score was 6.86±3.37 before the study which reduced insignificantly to 6.8±3.34 at the end of the study. In the control group, the mean 4C score increased insignificantly from 7.21±2.72 to 7.28±2.39. Furthermore, the result of the one-way analysis of covariance (ANCOVA) showed that after adjusting for baseline values, the differences between groups are not significant.
Conclusion:
Based on the results, this study showed no significant effect of vitamin A on COVID-19 severity.
Keywords: Vitamin A, Mortality, COVID-19
Copyright and License Information
© 2023 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The COVID-19 pandemic1 has affected about 767 million cases worldwide, and it is the underlying cause of death of more than six million people.2 In some patients, COVID-19 is associated with severe symptoms that lead to hospitalization.3 The severity of the disease is somehow attributed to the nutrition status. Previously, micronutrient deficiencies were associated with more severe viral infections.4 It has been found that micronutrient deficiencies could have an impact on immune response and severity of COVID-19.5,6 Vitamin A deficiency is one of the micronutrient deficiencies that may be associated with viral infections and pneumonia.4 Earlier studies indicated that vitamin A can increase immune functions by increasing the proliferation of lymphocytes and T-helper cells.4 Some research has been conducted to assess the effect of vitamin A supplementation on pneumonia in children and showed that this vitamin can decrease the severity of diseases7,8; however, other research did not reveal any significant outcome.9 In patients with COVID-19, Rohani et al observed that a 10-day supplementation of vitamin A can improve some clinical and paraclinical symptoms in patients.10 In another trial, Beigmohammadi et al showed that supplementation with fat-soluble vitamins and vitamin C has a positive impact on inflammatory response and disease severity of intensive care unit-admitted patients with COVID-19.11,12
Considering the high prevalence of COVID-19 worldwide and owing to the fact that there is no effective treatment for it, the use of complementary treatments is highlighted.13 Considering that vitamin A has a positive role in infectious disease and since vitamin A has an immune-modulating effect, we hypothesized that this vitamin can have a positive effect on hospitalized patients with severe COVID-19.14 In our previous report, we examined the effect of vitamin A supplementation on the mortality rate in these patients, finding out that vitamin A supplementation did not affect mortality in hospitalized patients with COVID-19. We assumed that it may have a significant effect on disease severity in these patients. So, we aimed to investigate the impact of vitamin A injection on the severity of COVID-19 in admitted patients to the hospital.
Methods
In this pilot open-labeled randomized controlled clinical trial (RCT), the impact of vitamin A injection on disease severity was assessed in patients with COVID-19. The patients were recruited from the Imam-Reza hospital in Tabriz, Iran, by convenience sampling. To be included in the study, patients should be adults (aged over 18 years), the presence of COVID-19 should be confirmed by reverse transcription polymerase chain reaction (RT-PCR), and the patients should be ventilator-independent at the time of inclusion. The pregnant and lactating patients as well as the users of high-dose vitamin A in the last month were excluded from the study.
For conducting this clinical trial, we have approval from the Ethics Committee of Tabriz University of Medical Sciences [Ethics code: IR.TBZMED.REC.1398.1305]. Moreover, before the initiation of the interventions, all participants gave written informed consent. Further, the intervention protocol was registered in the Iranian Registry of Clinical Trials (identifier: IRCT20170117032004N3; https://www.irct.ir/trial/46564).
According to a previous study,15 a pilot study should have at least 15 participants in each arm. To allow drop-outs, we included 18 patients in each arm. A computer-based random list was used to allocate patients to vitamin A group or the control group. The random allocation process was conducted by a colleague who did not have any other role in the trial, and the convenience sampling method was employed.
In the present clinical trial, the dose of vitamin A was chosen according to an earlier study on adults (Maintenance dose of 50 000 units intramuscularly once a day for two weeks).16 Patients in intervention and control groups proceeded with conventional therapies, including hydroxychloroquine, antiviral agents, corticosteroids, and antibiotics. There were no significant differences between groups regarding the type of received common treatments. This study was open-labeled, and none of the involved participants and researchers were blinded to group allocation.
A skilled physician recorded the patients’ general information and clinical laboratory data. All participants were recruited on the first day of admission to the hospital, and the symptoms and medical procedures were checked daily. All patients were followed up until they were discharged from the hospital or died.
For predicting mortality risk, we used the Coronavirus Clinical Characterization Consortium (4C) score. This score includes the age, gender, number of associated diseases, breathing frequency, peripheral oxygenation, Glasgow Coma Scale, blood urea nitrogen, and C-reactive protein (CRP) levels. Using this score, the patients were classified into four risk groups: low-risk group (4C score of ≤ 4), intermediate-risk group (4C score of 4–8), high-risk (4C score of 9–14), and very high-risk (4C score of ≥ 15) group.
Statistical Analysis
The statistical analysis was conducted by SPSS version 22.0. The normality of the data was checked using the Kolmogorov-Smirnov test. Considering the normal distribution of data, we presented the numerical variables as the mean (standard deviation) and compared them between groups using an independent t test. In addition, the nominal and categorical variables were presented as frequency (%) and compared with chi-square tests. For comparing the disease severity after treatments between intervention and control groups, the one-way analysis of covariance (ANCOVA) was used considering the age, sex, and baseline values as cofactors. The intention-to-treat protocol was further used for statistical analysis, and a P value of ≤ 0.05 was considered as a cut-off for statistical significance
Results
As depicted in Figure 1, this study included 36 patients with COVID-19. In both groups, three patients were lost to follow-up. Considering the intention-to-treat protocol of the study, the data of 18 patients were analyzed.
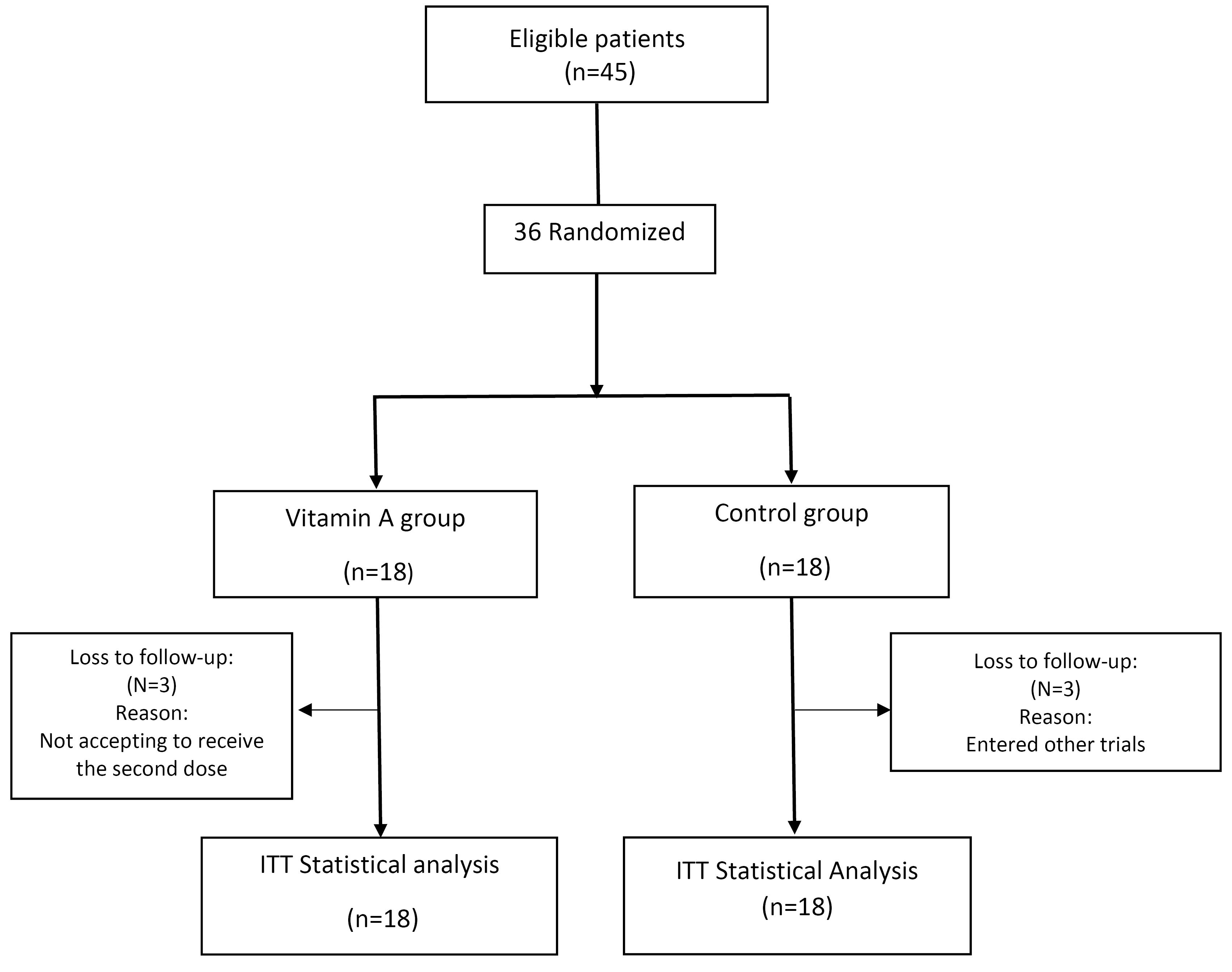
Figure 1.
Flow chart of patients recruitment and analysis. ITT: intention to treat
.
Flow chart of patients recruitment and analysis. ITT: intention to treat
The patients’ mean age was 61.43 ± 13.54 years, and 38.8% of these patients were female. As observed in Table 1, the baseline features of the patients were not significant between the groups.
Table 1.
Demographic and Clinical Characteristics of the Patients at Baseline
|
Variable
|
Vitamin A Group (n=18)
|
Control Group (n=18)
|
P
Value
|
| Age (y) |
59.94 ± 13.5 |
61.75 ± 12.8 |
0.69a |
| Male, No. (%) |
12 (66.6) |
10 (55.5) |
0.8b |
| Current smoker, No. (%) |
3 (16.6) |
3 (16.6) |
- |
| ICU admission, No. (%) |
4 (22.2) |
4 (22.2) |
|
| IMV, No. (%) |
3 (16.6) |
3 (16.6) |
|
| Initial vital signs and laboratory data, No. (%) |
| Number of comorbidities |
37.57 ± 0.53 |
37.42 ± 0.60 |
0.48b |
| GCS |
14.72 ± 0.66 |
14.75 ± 0.68 |
0.90a |
| RR |
27.46 ± 3.73 |
28.14 ± 3.65 |
0.62b |
| CRP (mg/L) |
2 ± 0.8 |
2.5 ± 0.85 |
0.16a |
| O2 saturation (%) |
83.50 ± 4.93 |
86.35 ± 3.46 |
0.06a |
| BUN (mg/dL) |
19.75 ± 11.35 |
16.01 ± 5.75 |
0.21a |
| C4 score |
6.86 ± 3.37 |
7.21 ± 2.71 |
0.76a |
Note. ICU: Intensive care unit; IMV: Invasive mechanical ventilation; GCS: Glasgow coma scale; RR: Respiratory rate; CRP: C-reactive protein; BUN: blood urea nitrogen.
aP value of independent t-test; bP value of chi-square.
Figure 2 indicates the mean 4C score in the intervention group and control group before and after the study. As can be seen, the mean 4C score in the intervention group was 6.86 ± 3.37 before the study which reduced insignificantly to 6.8 ± 3.34 at the end of the study. In the control group, the mean 4C score increased insignificantly from 7.21 ± 2.72 to 7.28 ± 2.39. The result of one-way ANCOVA displayed no significant differences between groups after adjusting for baseline values.
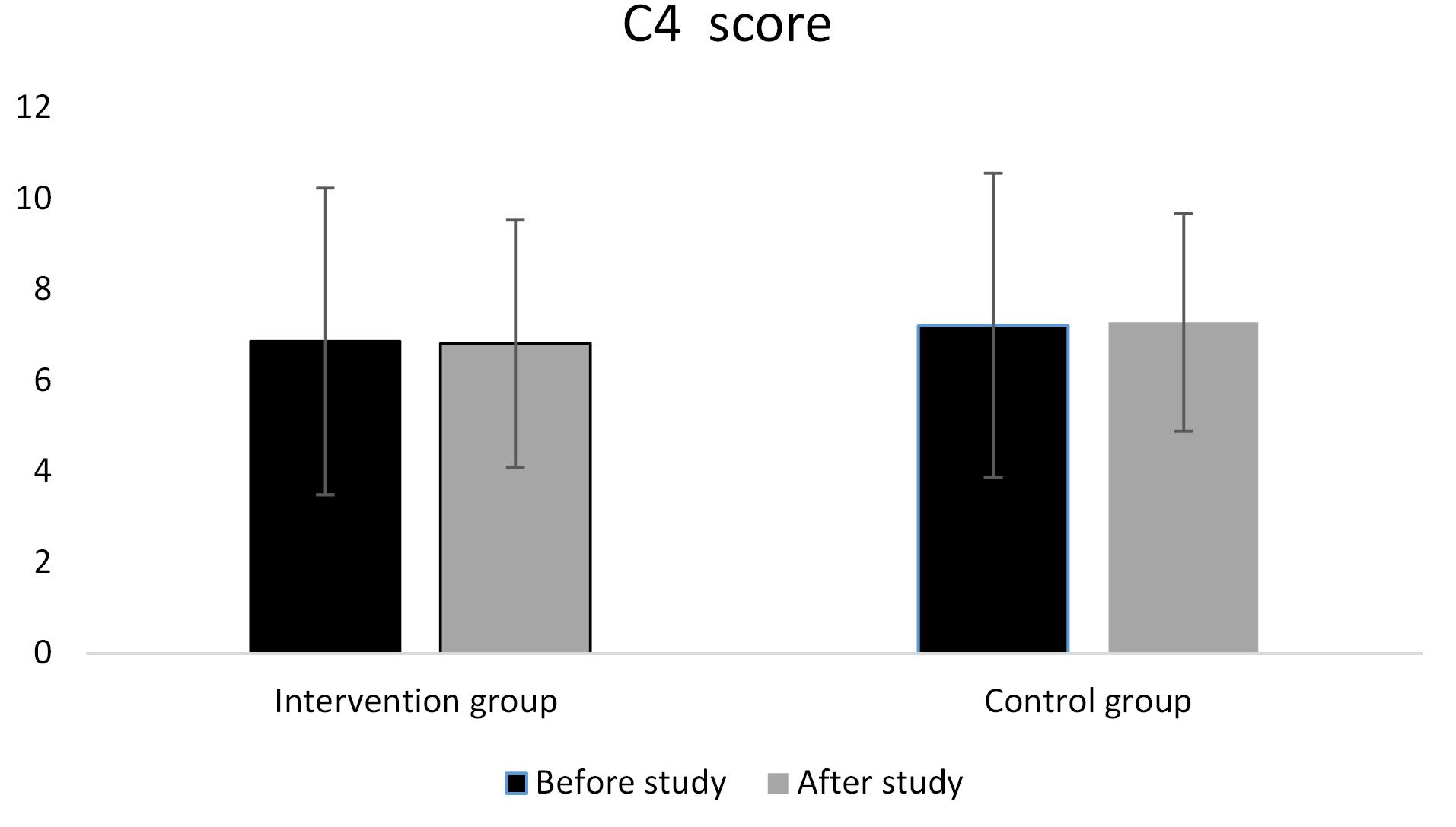
Figure 2.
Comparison of the 4C score between groups
*p value of one-way ANCOVA after adjusting for age, sex and baseline values
.
Comparison of the 4C score between groups
*p value of one-way ANCOVA after adjusting for age, sex and baseline values
Discussion
During the COVID-19 pandemic, different studies have focused on the use of different nutritional supplements to decrease mortality rates in these patients. This clinical trial investigated the effect of vitamin A injection on 4C score in patients with COVID-19 and showed that the disease severity decreased in the intervention group and increased in the control group. However, there were no significant differences between groups regarding severity scores. Previous studies on patients with COVID-19 indicated that supplementation with vitamin A can have positive effects on disease symptoms and inflammatory response in patients with COVID-19 that consequently decreases the severity of the disease.10,11 A meta-analysis of studies on patients with pneumonia also showed that vitamin A supplementation has no significant effect on the prevention of pneumonia.17 In addition, in patients with acute lower respiratory tract infections, vitamin A did not have a significant effect on disease severity.18 In contrast to these results, a study in children with pneumonia revealed the positive effect of vitamin A on decreasing the recovery period.19 We believed that the differences in the results of different studies could be attributed to the dissimilarities of the studied population. We studied the effect of vitamin A supplementation on adults; however, the studies that showed the positive effect of vitamin A were conducted on children. Moreover, previous studies were conducted on patients with pneumonia, while the present study’s population was patients with COVID-19. Serum vitamin A status and vitamin A dose could also affect the result.
One of the main limitations of the present clinical trial was the low sample size. Moreover, it was an unblinded study. To achieve more precise results, we suggested that future studies include more patients followed up the patients after discharge to study the after-discharge mortality.
Conclusion
Generally, the result of the present study showed that vitamin A supplementation had no significant effect on the disease severity in patients with COVID-19.
Ethics statement
The ethics committee of Tabriz University of Medical Sciences have approved the study protocol [Ethocs code: IR.TBZMED.REC.1398.1305].
Disclosure of funding source
This study was funded bytheLiver and Gastrointestinal Diseases Research Center, Tabriz University of Medical Sciences.
Conflict of interests declaration
The authors declare no conflict of interests.
Acknowledgments
The authors wish to thank the Liver and Gastrointestinal Diseases Research Center, Tabriz University of Medical Sciences for their financial support and epidemiological and statistical consultation.
Data availability statement
Data supported the result of this study will be available by request from corresponding author.
Consent for publication
None.
References
- Coronavirus Disease (COVID-19) Pandemic. Available from: https://www.who.int/europe/emergencies/situations/covid-19.
- Coronavirus Update. Available from: https://www.worldometers.info/coronavirus/.
- Martins-Filho PR, Tavares CSS, Santos VS. Factors associated with mortality in patients with COVID-19 A quantitative evidence synthesis of clinical and laboratory data. Eur J Intern Med 2020; 76:97-9. doi: 10.1016/j.ejim.2020.04.043 [Crossref] [ Google Scholar]
- Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr 2001; 21:167-92. doi: 10.1146/annurev.nutr.21.1.167 [Crossref] [ Google Scholar]
- Bae M, Kim H. Mini-review on the roles of vitamin C, vitamin D, and selenium in the immune system against COVID-19. Molecules 2020; 25(22):5346. doi: 10.3390/molecules25225346 [Crossref] [ Google Scholar]
- Pereira M, Dantas Damascena A, Galvão Azevedo LM, de Almeida Oliveira T, da Mota Santana J. Vitamin D deficiency aggravates COVID-19: systematic review and meta-analysis. Crit Rev Food Sci Nutr 2022; 62(5):1308-16. doi: 10.1080/10408398.2020.1841090 [Crossref] [ Google Scholar]
- Li R, Wu K, Li Y, Liang X, Tse WKF, Yang L. Revealing the targets and mechanisms of vitamin A in the treatment of COVID-19. Aging (Albany NY) 2020; 12(15):15784-96. doi: 10.18632/aging.103888 [Crossref] [ Google Scholar]
- Zhou W, Zuo X, Li J, Yu Z. Effects of nutrition intervention on the nutritional status and outcomes of pediatric patients with pneumonia. Minerva Pediatr 2016; 68(1):5-10. [ Google Scholar]
- Nacul LC, Arthur P, Kirkwood BR, Morris SS, Cameiro AC, Benjamin AF. The impact of vitamin A supplementation given during a pneumonia episode on the subsequent morbidity of children. Trop Med Int Health 1998; 3(8):661-6. doi: 10.1046/j.1365-3156.1998.00259.x [Crossref] [ Google Scholar]
- Rohani M, Mozaffar H, Mesri M, Shokri M, Delaney D, Karimy M. Evaluation and comparison of vitamin A supplementation with standard therapies in the treatment of patients with COVID-19. East Mediterr Health J 2022; 28(9):673-81. doi: 10.26719/emhj.22.064 [Crossref] [ Google Scholar]
- Beigmohammadi MT, Bitarafan S, Hoseindokht A, Abdollahi A, Amoozadeh L, Soltani D. The effect of supplementation with vitamins A, B, C, D, and E on disease severity and inflammatory responses in patients with COVID-19: a randomized clinical trial. Trials 2021; 22(1):802. doi: 10.1186/s13063-021-05795-4 [Crossref] [ Google Scholar]
- Amini K, Mojtahedzadeh M, Najafi A, Sharifnia H, Tabatabaei Mohammadi A. Effect of vitamin C on coagulation factors and endothelium function in patients with sepsis. Front Emerg Med 2023; 7(2):e16. doi: 10.18502/fem.v7i2.12768 [Crossref] [ Google Scholar]
- Carr AC. A new clinical trial to test high-dose vitamin C in patients with COVID-19. Crit Care 2020; 24(1):133. doi: 10.1186/s13054-020-02851-4 [Crossref] [ Google Scholar]
- Stephensen CB, Lietz G. Vitamin A in resistance to and recovery from infection: relevance to SARS-CoV2. Br J Nutr 2021; 126(11):1663-72. doi: 10.1017/s0007114521000246 [Crossref] [ Google Scholar]
- Whitehead AL, Julious SA, Cooper CL, Campbell MJ. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat Methods Med Res 2016; 25(3):1057-73. doi: 10.1177/0962280215588241 [Crossref] [ Google Scholar]
- Said E, Mousa S, Fawzi M, Sabry NA, Farid S. Combined effect of high-dose vitamin A, vitamin E supplementation, and zinc on adult patients with diabetes: A randomized trial. J Adv Res 2021; 28:27-33. doi: 10.1016/j.jare.2020.06.013 [Crossref] [ Google Scholar]
- Sommer A, Rahmathullah L, Underwood B, Milton R, Reddy V, West K. Potential interventions for the prevention of childhood pneumonia in developing countries: a meta-analysis of data from field trials to assess the impact of vitamin A supplementation on pneumonia morbidity and mortality The Vitamin A and Pneumonia Working Group. Bull World Health Organ 1995; 73(5):609-19. [ Google Scholar]
- Ni J, Wei J, Wu T. Vitamin A for non-measles pneumonia in children. Cochrane Database Syst Rev 2005; 2005(3):CD003700. doi: 10.1002/14651858.CD003700.pub2 [Crossref] [ Google Scholar]
- Si NV, Grytter C, Vy NN, Hue NB, Pedersen FK. High dose vitamin A supplementation in the course of pneumonia in Vietnamese children. Acta Paediatr 1997; 86(10):1052-5. doi: 10.1111/j.1651-2227.1997.tb14805.x [Crossref] [ Google Scholar]